Figures & data
Figure 1 Flow diagram of the patients with hepatocellular carcinoma.
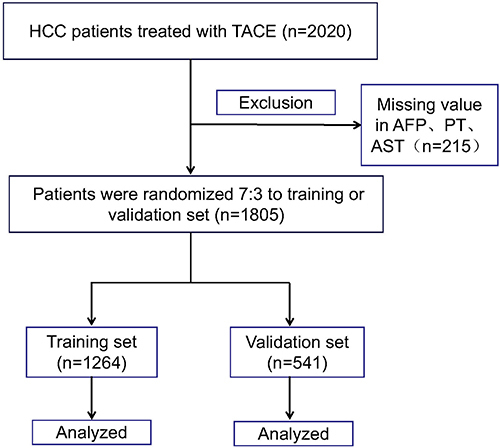
Table 1 Baseline Characteristics of Patients in the Training and Validation Sets (N=1805)
Table 2 Results of Univariate and Multivariate Analysis in the Training Set
Figure 2 The OS according to the ALFP score in the training and validation sets. (A) The median OS according to the ALFP score in the training set was as follows: 0 points (n = 112), 50.1 months (95% CI not applicable); 1 point (n = 484), 33.6 months (95% CI 27.4–39.8); 2 points (n = 540), 20.4 months (95% CI 18.0–22.8); and 3 points (n = 128), 12.7 months (95% CI 10.2–15.2); this amounted to a statistically significant difference (p < 0.001). (B) The median OS according to the ALFP score in the validation set was as follows: 0 points (n = 54), 42.5 months (95% CI 16.1–68.9); 1 point (n = 188), 32.6 months (95% CI 25.1–40.1); 2 points (n = 240), 20.4 months (95% CI 16.4–24.4); and 3 points (n = 59), 13.0 months (95% CI 0.0–27.3); this amounted to a statistically significant difference (p = 0.0019).
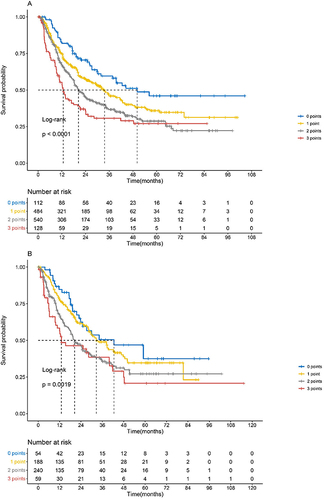
Figure 3 The OS according to the ALFP score in the training and validation sets stratified by the diameter of tumor. (A) The median OS according to the ALFP score in the training set stratified by the diameter of tumor was as follows: The diameter of tumor ≤5 cm group, 0 points (n = 52), not applicable (95% CI not applicable); 1 point (n = 170), 54.9 months (95% CI not applicable); 2 points (n = 162), 50.0 months (95% CI 38.5–61.5); and 3 points (n = 44), 23.9 months (95% CI 12.3–35.5); this amounted to a statistically significant difference (p = 0.0077). The diameter of tumor >5 cm group, 0 points (n = 60), 31.8 months (95% CI 11.7–51.9); 1 point (n = 314), 20.3 months (95% CI 17.4–23.2); 2 points (n = 378), 15.6 months (95% CI 13.1–18.1); and 3 points (n = 84), 10.8 months (95% CI 8.2–13.4); this amounted not to a statistically significant difference (p < 0.001). (B) The median OS according to the ALFP score in the validation set stratified by the diameter of tumor was as follows: The diameter of tumor ≤5 cm group, 0 points (n = 26), 59.0 months (95% CI 38.8–79.2); 1 point (n = 69), 47.7 months (95% CI not applicable); 2 points (n = 70), 29.6 months (95% CI 19.1–40.1); and 3 points (n = 24), 27.0 months (95% CI 0.0–54.1); this amounted to a statistically significant difference (p = 0.015). The diameter of tumor >5 cm group, 0 points (n = 28), 21.6 months (95% CI 14.5–28.7); 1 point (n = 119), 25.1 months (95% CI 16.5–33.7); 2 points (n = 170), 16.3 months (95% CI 11.8–20.8); and 3 points (n = 35), 9.8 months (95% CI 2.8–16.8); this amounted not to a statistically significant difference (p = 0.092).
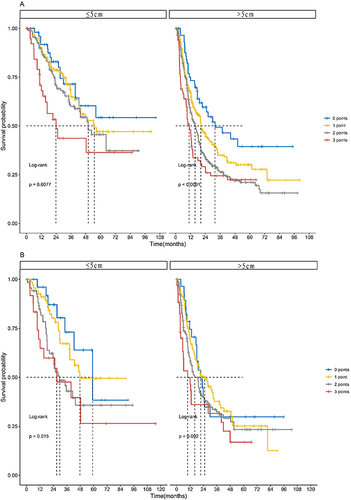
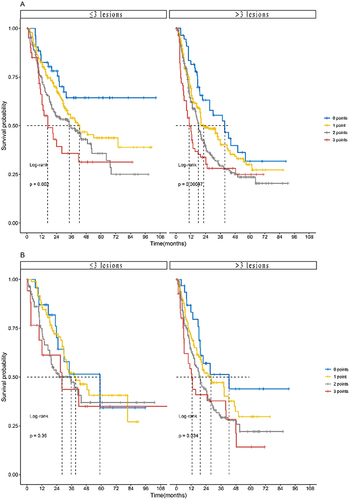
Figure 4 The OS according to the ALFP score in the training and validation sets stratified by the No. of intrahepatic lesions. (A) The median OS according to the ALFP score in the training set stratified by the No. of intrahepatic lesions was as follows: The No. of intrahepatic lesions ≤3 group, 0 points (n = 54), not applicable (95% CI not applicable); 1 point (n = 191), 42.7 months (95% CI 27.4–58.0); 2 points (n = 201), 34.4 months (95% CI 21.4–47.4); and 3 points (n = 41), 16.8 months (95% CI 6.8–26.8); this amounted to a statistically significant difference (p = 0.002). The No. of intrahepatic lesions >3 group, 0 points (n = 58), 39.5 months (95% CI 23.6–55.4); 1 point (n = 293), 22.4 months (95% CI 14.5–30.3); 2 points (n = 339), 18.2 months (95% CI 16.0–20.4); and 3 points (n = 87), 10.8 months (95% CI 7.8–13.8); this amounted not to a statistically significant difference (p < 0.001). (B) The median OS according to the ALFP score in the validation set stratified by the No. of intrahepatic lesions was as follows: The No. of intrahepatic lesions ≤3 group, 0 points (n = 23), 59.0 months (95% CI 28.2–89.8); 1 point (n = 87), 39.3 months (95% CI 24.3–54.3); 2 points (n = 87), 35.6 months (95% CI 15.3–55.9); and 3 points (n = 17), 28.3 months (95% CI 3.0–53.6); this amounted not to a statistically significant difference (p = 0.35). The No. of intrahepatic lesions >3 group, 0 points (n = 31), 42.5 months (95% CI 7.9–77.1); 1 point (n = 101), 28.0 months (95% CI 11.2–44.8); 2 points (n = 152), 19.2 months (95% CI 15.1–23.3); and 3 points (n = 42), 12.5 months (95% CI 8.3–16.7); this amounted not to a statistically significant difference (p = 0.034).