Figures & data
Figure 1 Patient flowchart. A patient might meet several exclusion criteria, but they were excluded only once from the uppermost criteria.
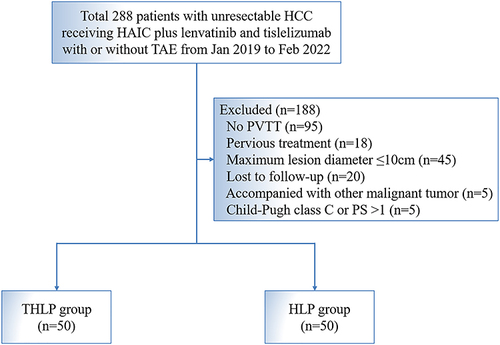
Table 1 Baseline Characteristics of Patients in Two Groups
Table 2 Tumor Response in Two Groups
Figure 2 Kaplan-Meier curves of (A) overall survival in all patients; (B) progression-free survival in all patients (C) overall survival in two groups; and (D) progression-free survival in two groups.
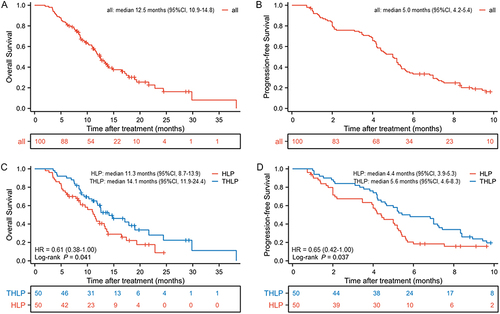
Figure 3 Kaplan-Meier curves of overall survival between two groups in patients (A) with PVTT I/II; (B) with PVTT III/IV; (C) without extrahepatic metastasis; (D) with extrahepatic metastasis; (E) with AFP ≥400 ng/mL and (F) with AFP <400 ng/mL.
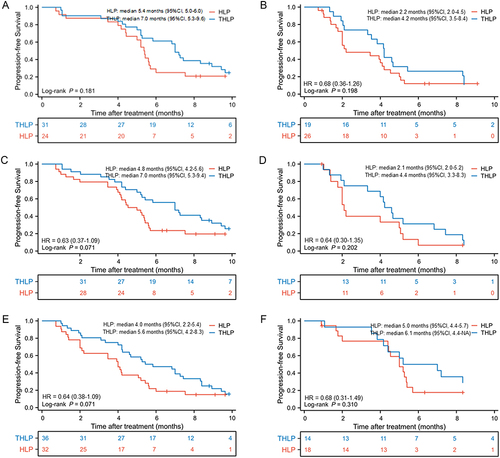
Figure 4 Kaplan-Meier curves of progression-free survival between two groups in patients (A) with PVTT I/II; (B) with PVTT III/IV; (C) without extrahepatic metastasis; (D) with extrahepatic metastasis; (E) with AFP >400 ng/mL and (F) with AFP ≤400 ng/mL.
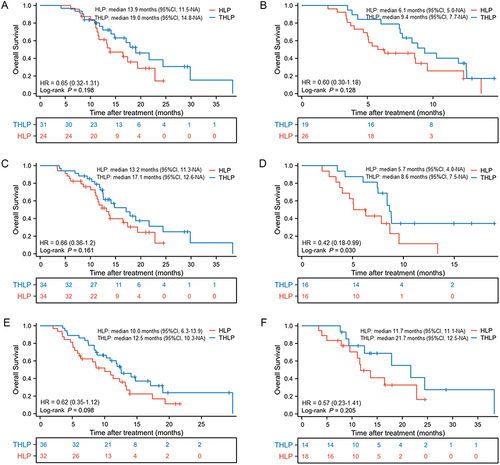
Table 3 Univariate and Multivariate Analysis of Risk Factors for Overall Survival and Progression-Free Survival
Table 4 Treatment-Related Adverse Events
