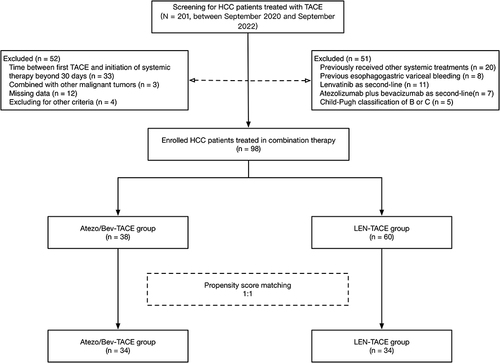
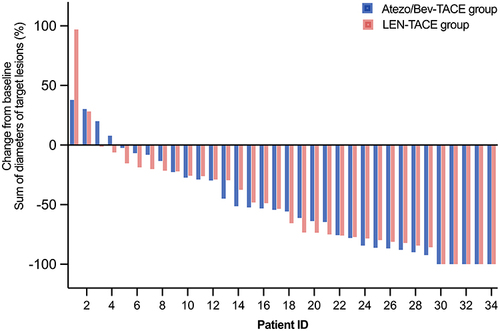
Figures & data
Table 1 Baseline Characteristics of Patients Before Propensity Score Matching
Table 2 Baseline Characteristics of Patients After Propensity Score Matching
Figure 2 Kaplan–Meier curves showing OS (A), PFS (B) and DOR (C) of each group.
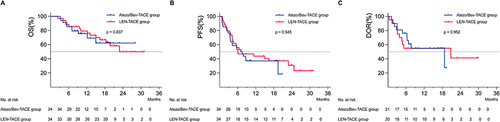
Table 3 Summary of Best Overall Response
Figure 4 Forest plots with subgroup analysis for factors associated with (a) PFS and (b) OS.
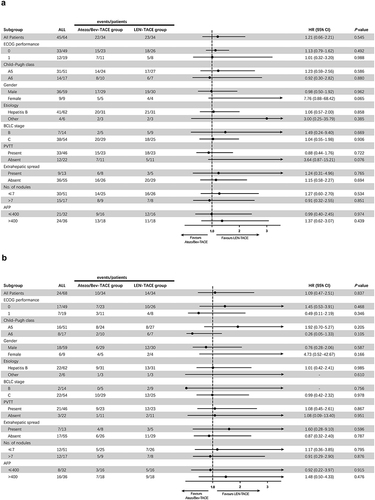
Table 4 Safety Profiles and Adverse Events