Figures & data
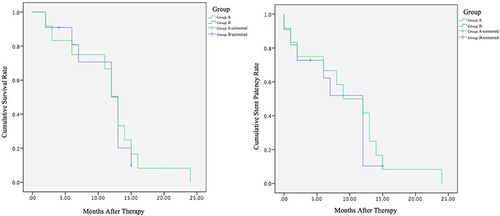
Figure 1 Study flowchart.
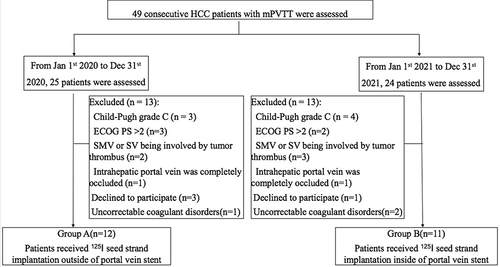
Figure 2 A 68-year-old male HCC patient with mPVTT underwent 125I seed strand (30 seeds) implantation inside the portal vein stent. The patient underwent transarterial chemoembolization (TACE) 20 days later and died 6 months after the 125I seed strand and portal vein stent implantation because of liver failure caused by progression of the tumor. (a) Portography shows a large filling defect in the main portal vein and the right branch of the portal vein with near-complete obstruction of the portal venous blood flow. (b) Portography after deployment of the portal vein stent shows restored blood flow in the main portal vein and the right branch. (c and d) Ultrasound images showing a portion of the 125I seed strand in the liver parenchyma and the blood flow in the patent portal vein stent. (e) SPECT imaging shows homogeneous radiation dose distribution from the 125I seed strand covering the range of the tumor thrombus. (f) Follow-up computed tomography at 3 months after the procedure shows the 125I seed strand inside the stent without displacement, with steady blood flow in the stent.
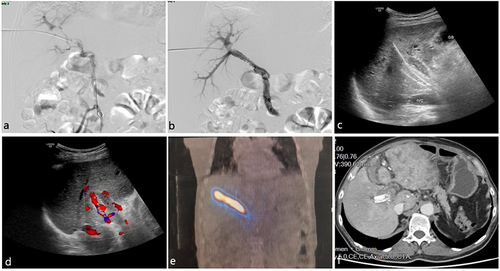
Figure 3 A 67-year-old female patient with HCC and mPVTT underwent 125I seed strand (26 seeds) implantation inside the portal vein stent. The patient had 3 transarterial chemoembolization (TACE) treatments during a 13-month follow-up, resulting in a stable partial response (PR). (a and b) Before treatment, the enhanced computed tomography shows the tumor thrombus in the main portal vein and the right branch of portal vein (arrow). (c) During the stent/strand placement, initial portography shows the filling defect in the portal vein with portal vein stenosis. (d) Portography following deployment of the portal vein stent shows restored blood flow in the main portal vein and the right branch. (e and f) Follow-up computed tomography at 1 year after the procedure shows the 125I seed strand inside the stent without displacement, and steady blood flow in the stent (arrow).
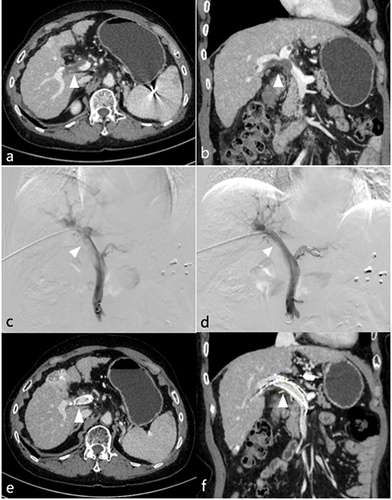
Table 1 Characteristics of the Study Population
Table 2 Pre- and Post-Procedure Liver Function