Figures & data
Table 1 In vitro binding parameters of ixekizumab to human, cynomolgus monkey, and rabbit IL-17A determined using surface plasmon resonance
Figure 1 Ixekizumab binds specifically to IL-17A.
Notes: Human IL-17 family member proteins, mouse IL-17A, and human IL-22 were coated into individual ELISA plate wells. Ixekizumab was then added at varying concentration up to 10 µg/mL. The absorbance at 450 nm represents the average values and standard error obtained from duplicate determinations at each concentration of ixekizumab bound to the test proteins.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; IL, interleukin.
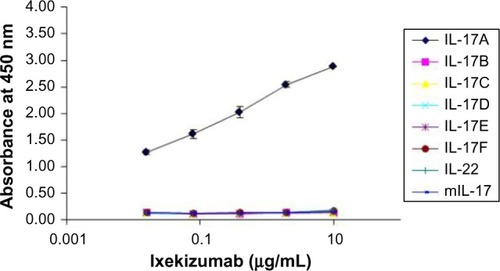
Figure 2 Ixekizumab competes with IL-17RA binding to immobilized human IL-17A.
Notes: Human IL-17A was immobilized on a Biacore chip surface. Binding of IL-17RA/Fc to immobilized IL-17A was shown as increase in response units upon injection of IL-17RA/Fc (A). Ixekizumab bound to immobilized IL-17A as shown by the increase in response units following injection (B). Upon binding of ixekizumab to IL-17A, IL-17RA/Fc can no longer bind (B).
Abbreviations: IL, interleukin; RU, response units; Resp Diff, the difference in RU between active flow cell and reference flow cell.
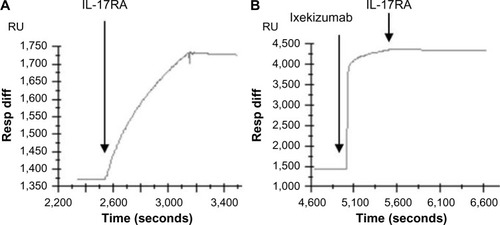
Figure 3 Ixekizumab neutralizes human IL-17A, human IL-17A/F heterodimer, and cynomolgus monkey IL-17A.
Notes: (A) HT-29 cells were treated with a dose range of human IL-17A or IL-17A/F for ∼48 hours. GROα was measured in the culture medium by ELISA. (B) HT-29 cells were treated with a constant amount of either human IL-17A (60 ng/mL =1,875 pM) or human IL-17A/F (1,000 ng/mL =32,573 pM), in the presence of either ixekizumab or control human IgG4 at the indicated concentrations. After ∼48 hours, GROα in the culture media was measured by ELISA. (C) HT-29 colon cancer cells were treated with a constant amount of cynomolgus monkey IL-17 (60 ng/mL), in the presence of either ixekizumab or control human IgG4 at the indicated concentrations for 48 hours, and GROα in the culture media measured by ELISA. Results in (A) and (B) are shown as the mean of triplicate treatments ± SD and are representative of seven independent experiments. Results in (C) are shown as the mean of triplicate treatments ± SD and are representative of two independent experiments. Human and cynomolgus monkey IL-17A were tested for neutralization in separate experiments.
Abbreviations: cyno, cynomolgus; ELISA, enzyme-linked immunosorbent assay; IL, interleukin; SD, standard deviation; GRO, human growth-regulated oncogene.
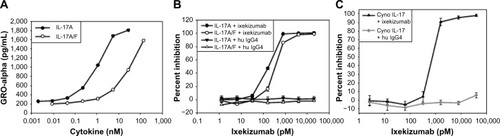
Figure 4 Ixekizumab epitope.
Notes: (A) 4T1 cells were treated with a constant amount of human IL-17A wild type, mutant h1, or vector control supernatant in the presence of increasing amounts of ixekizumab (closed symbols) or isotope control antibody (open symbols). After 48 hours, KC in the supernatant was measured by ELISA. Results are shown as the mean of triplicate treatments ± SD and are representative of four independent experiments. (B) Structure of the IL-17A:IL-17RA complex (4hsa) with key amino acid residues in the epitope for ixekizumab highlighted. IL-17RA is colored in magenta, and the IL-17A dimer subunits are colored in cyan and green. The key epitope region (DGNVDYH) in IL-17A for ixekizumab is highlighted in yellow or brown in each subunit of the cytokine. This figure was generated using the PyMOL Molecular Graphics System (Version 1.7.0.3; Schrödinger, LLC).
Abbreviations: ELISA, enzyme-linked immunosorbent assay; IL, interleukin; SD, standard deviation; KC, keratinocyte chemoattractant.
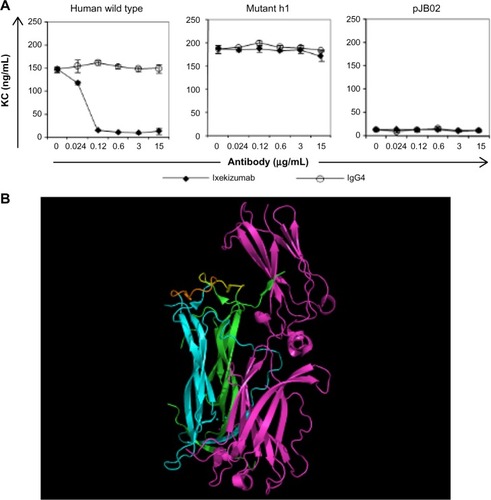
Figure 5 Ixekizumab inhibits human IL-17A-induced KC secretion in a mouse PD model.
Notes: Human IL-17A (3 µg) was administered to mice SC 1 hour after IV injection of 1 mg/kg, 0.1 mg/kg, 0.01 mg/kg, or 0.001 mg/kg ixekizumab (corresponding to 20 µg, 2 µg, 0.2 µg, and 0.02 µg per mouse, respectively). KC levels were determined by ELISA 2 hours posthuman IL-17A injection. n=5 mice per group. One-way ANOVA was used for the pairwise comparison of KC chemokine levels between treatments. *The unadjusted P-value is 0.0012 comparing the IL-17A +20 µg control mAb group and the IL-17A +20 µg ixekizumab group. The figure represents one experiment of two separate studies.
Abbreviations: SC, subcutaneous; ANOVA, analysis of variance; ELISA, enzyme-linked immunosorbent assay; IL, interleukin; IV, intravenous; KC, keratinocyte chemoattractant; mAb, monoclonal antibody; PD, pharmacodynamic.
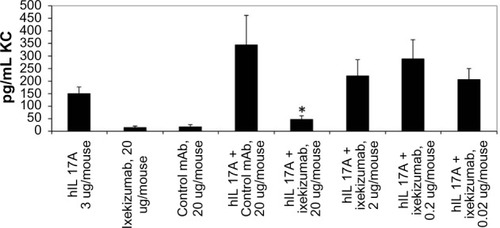
Figure S1 Ixekizumab humanization.
Notes: (A) Variable region sequence alignment of selected human germline genes, VH1–69 and VκA18, parental mouse antibody 2321, and ixekizumab. Germline homologous residues of 2321 are indicated by a dash. CDR residues are underlined, and Kabat antibody numbering system used. The CDR residues that differ from VH1–69 or VκA18 germline sequence are shown in green (different from VH1–69 or VκA18 but present in other human germline sequences) and red (not present in any human germline sequences). (B) Calculation of homology to human germline. ‡Excluding HCDR3 and *including CDR residues present at the homologous location in other human germline genes.
Abbreviations: CDR, complementarity-determining region; mAb, monoclonal antibody; VH, variable heavy; Vκ, variable kappa; N/A, not applicable.
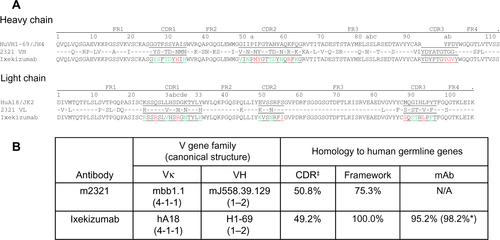
Figure S2 Ixekizumab epitope mapping.
Notes: (A) Mutagenesis of the six regions of the potential binding sites. (B) 4T1 cells were treated with a constant amount of human IL-17A wild type, mutant, or vector control supernatant in the presence of increasing amounts of ixekizumab (closed symbols) or isotope control antibody (open symbols). After 48 hours, KC in the supernatant was measured by ELISA. Results are shown as the mean of triplicate treatments ± standard deviation and are representative of four independent experiments.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; IL, interleukin; KC, keratinocyte chemoattractant.
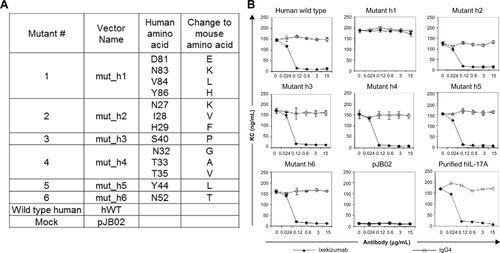
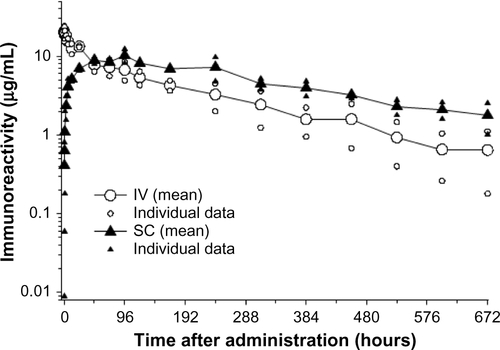
Figure S3 Serum pharmacokinetic profile of ixekizumab in male cynomolgus monkeys.
Notes: Following IV administration of 1 mg/kg, ixekizumab was eliminated with a mean half-life of 6.5 days. After SC administration of 1 mg/kg, it reached an average maximal plasma concentration of 11.1 µg/mL ∼72 hours postdose. The mean elimination half-life following subcutaneous injection was 10.3 days. The figure illustrates mean curve and data from individual animals (n=2).
Abbreviations: IV, intravenous; SC, subcutaneous.