Figures & data
Figure 1 2-DG (mM) depletes intracellular ATP and inhibits in vitro capillary-like structure formation in HBMEC. In order to assess the impact of ATP requirement for in vitro tubulogenesis, HBMEC were seeded on top of Matrigel as described in the Methods section and left to form structures. A) Capillaries were then treated with 2-DG (mM) or Mannose (mM). B) The impact of 2-DG was also monitored during the formation of the structures, where cells were treated with various concentrations of 2-DG 30 min after seeding of the cells on top of Matrigel, and structure formation monitored after 18 h. Representative phase contrast pictures were taken. C) The extent of three-dimensional capillary-like structure formation (tubulogenesis) and of cell survival was assessed as described in the Methods section. D) Intracellular ATP as well as total protein content were assessed as described in the Methods section in vehicle- or 2-DG-treated cells. The length of the tube network was quantitated using Northern Eclipse software.
Notes: Values are means of two independent experiments (*P < 0.01 versus control alone); bars, ± SD.
Abbreviations: HBMEC human brain microvasular endothelial cell; ATP, adenosine triphosphate; 2-DG, 2 deopy-D-glucose.
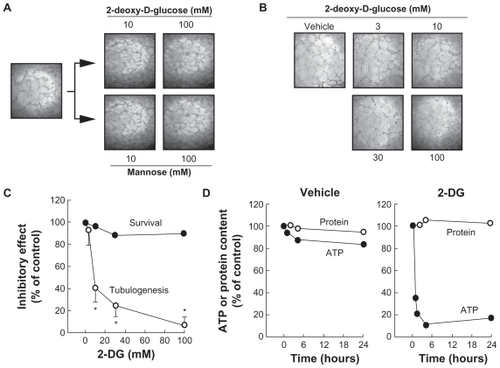
Figure 2 2-DG inhibits PMA-induced MMP-9 secretion in HBMEC. HBMEC were serum-starved in the presence of various concentrations of 2-DG in combination with vehicle or 1 μM PMA for 18 h. A) Conditioned media were then harvested and gelatin zymography was performed in order to detect proMMP-9 and proMMP-2 hydrolytic activity as described in the Methods section. B) Scanning densitometry was used to quantify the extent of either basal proMMP-2 gelatin hydrolysis (open circles), or proMMP-9 (closed circles) in PMA-treated cells.
Note: Data shown are representative of two independent experiments.
Abbreviations: 2-DG, 2-dexy-D-glucose; HBMEC, mitovasuila endothelial cells; MMP, matrix metalloproteinase; PMA, phorbol 12-myristate 13-acetate; GA DPH, glyceraldetyde 3- phisiphate dehydrogenase; PAGE, pohyacrylanide electrophoresis.
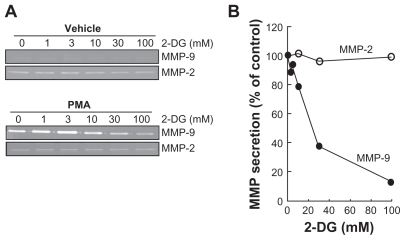
Figure 3 2-DG inhibits PMA-induced IκB phosphorylation that leads to IκB degradation. A) HBMEC were serum-starved for 30 min in the presence of either vehicle or 100 mM 2-DG. Cells were then incubated for the indicated time with 1 μM PMA. Lysates were isolated, electrophoresed via SDS-PAGE and immunodetection of phosphorylated IκB (P-IκB), IκB, and of GA PDH proteins was performed as described in the Methods section. B) Quantification was performed by scanning densitometry of the autoradiograms. Data were expressed as x-fold induction over basal untreated cells of the P-IκB/IκB ratios in vehicle pre-treated cells (open circles) and 2-DG pre-treated cells (closed circles).
Abbreviations: 2-DG, 2-dexy-D-glucose; HBMEC, mitovasuila endothelial cells; MMP, matrix metalloproteinase; PMA, phorbol 12-myristate 13-acetate; GA DPH, glyceraldetyde 3- phisiphate dehydrogenase; PAGE, pohyacrylanide electrophoresis.
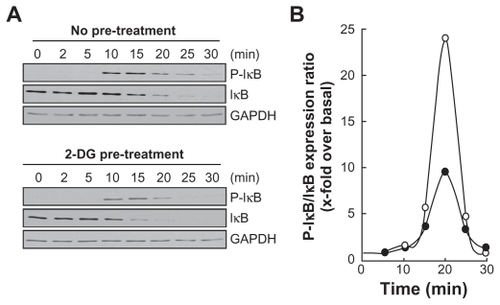
Figure 4 2-DG inhibits PMA-induced nuclear translocation of the p65 subunit of NF-κB. A) HBMEC were serum-starved for 30 min in the presence of either vehicle or 100 mM 2-DG. Cells were then incubated for the indicated time with 1 μM PMA. Nuclear extracts were isolated, electrophoresed via SDS-PAGE and immunodetection of the p65 subunit of NF-κB protein was performed as described in the Methods section. B) Quantification was performed by scanning densitometry of the autoradiograms. Data were expressed as x-fold induction over basal untreated cells of the vehicle pretreated cells (open circles) and 2-DG pre-treated cells (closed circles).
Abbreviations: HBMEC, mitovasuila endothelial cells; PAGE, pohyacrylanide electrophoresis. PMA, phorbol 12-myristate 13-acetate; 2-DG-glucose.
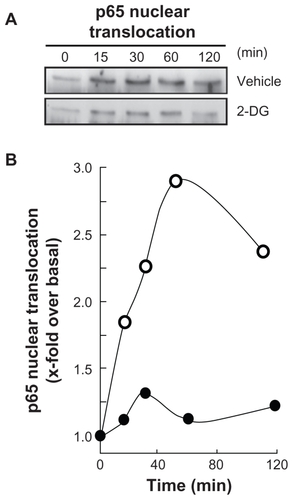
Figure 5 2-DG potentiates PMA-induced cyclooxygenase-2 and GRP78 expression. A) HBMEC were serum starved for 18 h in the presence or absence of 1 μM PMA and in combination with either 30 mM 2-DG or 30 mM Man. Lysates were isolated, electrophoresed via SDS-PAGE, and immunodetection of COX-2, GRP78, and GA PDH was performed as described in the Methods section. B) HBMEC were treated as in (A) with various doses of 2-DG. Lysates were isolated, electrophoresed via SDS-PAGE, and immunodetection of COX-2, GRP78, and GA PDH was performed as described in the Methods section. C) Scanning densitometry of COX-2 expression was only performed in PMA-treated cells since no COX-2 was detectable in vehicle-treated cells. D) Scanning densitometry of GRP78 expression was performed in vehicle- and in PMA-treated cells.
Abbreviations: HBMEC, mitovasuila endothelial cells; PMA, phorbol 12-myristate 13-acetate; 2-DG-glucose; PAGE, pohyacrylanide electrophoresis; GA DPH, glyceraldetyde, 3-phisiphate; man, mannose.
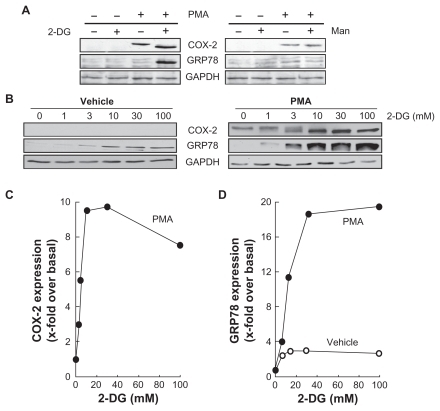
Figure 6 Increased endoplasmic reticulum stress, rather than MMP-mediated EC M hydrolysis, correlates with cyclooxygenase-2 expression in HBMEC. The effects of PMA treatments in the presence of increasing 2-DG concentrations were plotted in order to assess any correlation between A) ER stress (GRP78 expression) and inflammation (COX-2 expression), and B) between EC M hydrolysis (MMP-9 expression) and inflammation.
Abbreviations: EC M,extracellular matrix; MMP, matrix metallo prsteinase; HBMEC, mitovasuila endothelial cells; PMA, phorbol 12-myristate 13-acetate; 2-DG-glucose; ER, endoplasmic reticulam.
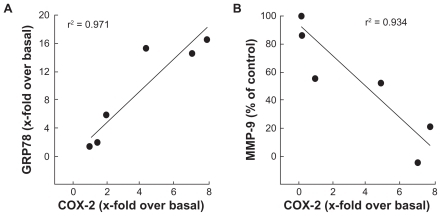
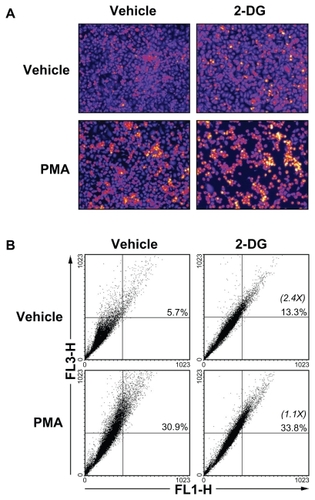
Figure 7 2-DG induced autophagy is abrogated in PMA-treated HBMEC. Autophagy was assessed as described in the Methods section in vehicle, 100 mM 2-DG-, 1 μM PMA-, and 100 mM 2-DG/1 μM PMA-treated HBMEC. A) Representative microphotographs of the formation of acidic vesicular organelles were taken upon acridine orange staining. B) The extent of autophagy was further confirmed by flow-cytometry of acridine orange stained cells and the percent of autophagy indicated for each condition. In between parenthesis, the extent of autophagy induction is indicated.
Abbreviations: HBMEC, mitovasuila endothelial cells; PMA, phorbol 12-myristate 13-acetate; 2-DG-glucose.