Figures & data
Figure 1 Pathways of T cell interactions with commensals. Depicted are commensal bacteria in the lumen of the intestine. Intraepithelial T cells are situated within the epithelial layer and can interact directly with commensals. T cells in mesenteric lymph nodes, the lamina propria, or Peyer’s patches rely on signaling by other cell types. Dendritic cells can traffic to the T cell sites and bring commensal antigens. The intestinal epithelium can also produce immunogenic peptides that influence production of T cells in the non-epithelial sites.
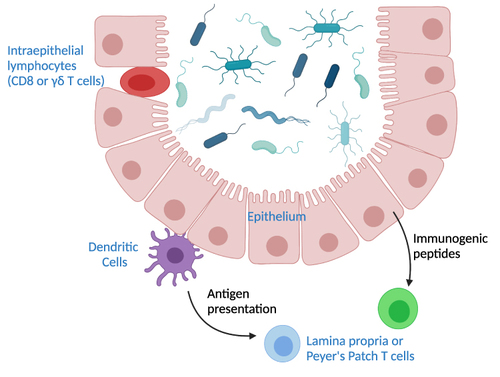
Figure 2 C. albicans as the major inducer of intestinal Th17 cells, thereby mediating broad range anti-fungal immunity. C. albicans specific Th17 cells secrete IL-17 family cytokines, that promote barrier stability, AMP production and mediate immunity against other commensal fungi species. Additionally, it has been proposed that Th17 cells modulate neutrophil function, improving defense against commensals such as S. aureus. C. albicans has also been reported to induce a Treg population, which might help Th17 differentiation, as well as smaller Th1 and Th2 populations of yet unknown activity. Proinflammatory and pathogenic functions of anti-fungal CSTCs arise typically with altered environmental signals, such as SAA 1+2 signaling or cross-reactivity towards pathogens such as A. fumigatus in the lung.
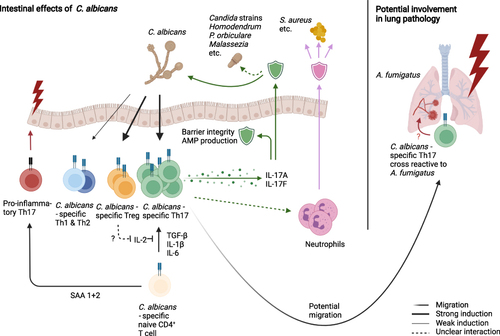
Figure 3 Induction of different T cell subsets by key commensal organisms. Commensal viruses stimulate IELs but might also be capable of inducing proliferation of classical cytotoxic CD8+ T cells. Commensal viruses, especially phages, have the potential to indirectly influence CSTC populations in the intestine by regulating commensal microorganism levels. While multiple commensals can directly or indirectly exhibit protective functions on epithelial cells, Lactobacillus species are a well-established example of this. In the context of antifungal immunity, Tregs are hypothesized to promote Th17 differentiation by IL-2 depletion. Generally, the induction of T cell populations depends not only on the type of commensal, but also on environmental signaling and inflammatory tissue state.
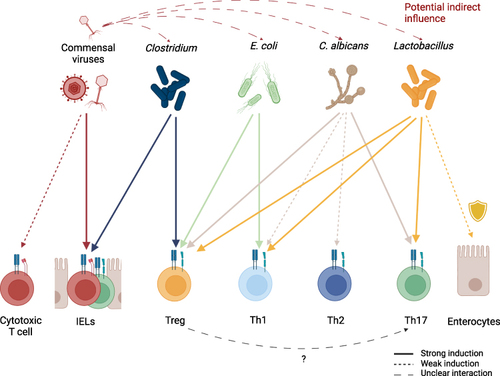
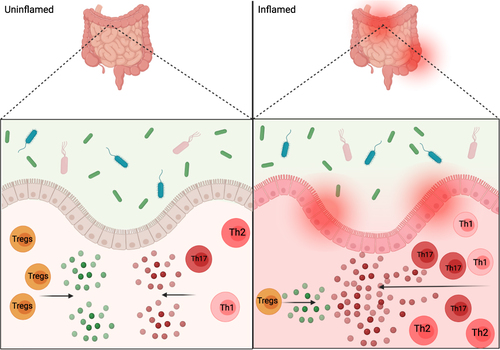
Figure 4 Intestinal inflammation and commensals. Depiction of proposed CSTC, and cytokine release, imbalance in inflamed gut versus the balanced anti-inflammatory and pro-inflammatory signals in an uninflamed gut. The interactions between commensals and gut epithelium help in promoting homeostasis, but could also play a role in gut pathogenesis and inflammation as depicted on inflamed side of figure.