Figures & data
Figure 1 Protein expression profile in manipulated HEK293 cell line and schematic depiction of Caspase 1 activation. (A) Full expression profile of proteins (TetR, ASC, LacZ, Pro-caspase-1, Cleaved-caspase-1, NLRP3 and house keeping gene GAPDH) in a range of manipulated HEK 293 cell lines in the presence or absence of 25 ng/mL Dox treatment for 24h; (B) schematic illustration of the CASP1/NLRP3-iASC-HEK293 model works in the presence or absence of Dox in the system. The TetR protein was expressed using a TetR-containing plasmid capable of interacting with the Tet responsive element (TRE) of the Tet operator (TetO) in the tetracycline-dependent promoter upstream of the target gene ASC, which interfered the expression of ASC protein although NLRP3/CASP1 simultaneously expressed stably in the cells, the inflammasome was not assembly (inactive). Once Dox is added in this system, it will bind to TetR and cause conformational changes in the repressor, making it unable to bind to the TetO. The TetR-Tet complex then dissociates from the TetO and allows induction of transcription from ASC. Increased ASC protein together with the high levels of NLRP3/CASP1 lead to the assembly of the Nlrp3 inflammasome. The activation of NLRP3 inflammasome subsequently cleaved pro-caspase 1 and initiated downstream reactions (active). Data is representative of three independent experiments. 1b was created with BioRender.com.
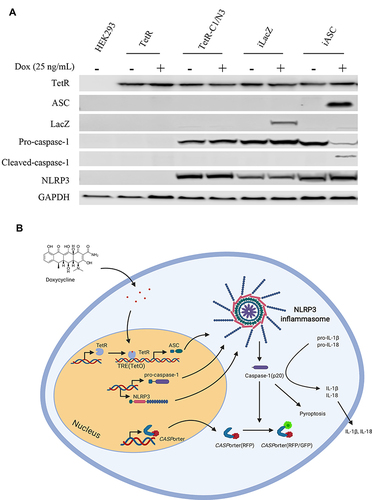
Figure 2 Co-immunoprecipitation (co-IP) of CASP 1 with NLRP3 in HEK293-iASC-NLRP3/CASP1 cells after Dox-induced ASC expression. HEK293-iASC-NLRP3/CASP1 or HEK293-iLacZ-NLRP3/CASP1 cells were seeded in six-well plates overnight and treated with Dox 25 ng/mL for 24h. Cell lysates were extracted and co-immunoprecipitation was performed using anti-NLRP3 antibody. Lysates (inputs) and immunoprecipitated proteins were analyzed by immunoblotting using CASP1, NLRP3 and GAPDH antibodies. GAPDH was used as a loading control for the inputs while iLacZ control cells (right 2 lanes) were used as the negative control for the co-immunoprecipitation experiments.
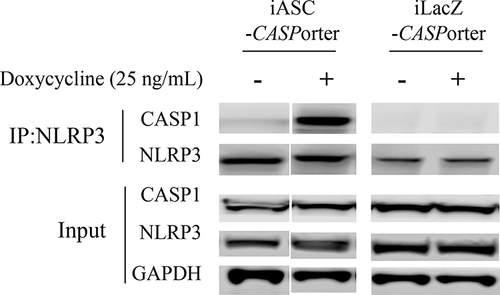
Figure 3 Schematic illustration of CASPorter generation in HEK293-iASC-NLRP3/CASP1 cells. (A) Scheme for the CASPorter and the GFP-based caspase-1 activity indicator (GC1A1); (B) cleavage site of GC1AI versus the reported GFP-based caspase-3 activity indicator (GC3AI); (C) schematic representation of the switch-on or switch-off of the GFP intensity (reflected by GFP:RFP ratio), indicating the inhibitory effect of small molecule inhibitors on the activation of the NALP3 inflammasome. (D) GFP intensity increased overtime with Dox 25 ng/mL treated for 24h, and co-treatment with the reported NLRP3 inhibitor MCC950 showed dose-dependent inhibition by in HEK293-NLRP3/CASP1-iASC cells but not in HEK293-NLRP3/CASP1-iLacZ cells. ##p < 0.001 compared to control, *p < 0.01 compared to Dox treatment at the same time point, **p < 0.001 compare to Dox treatment at the same time point.
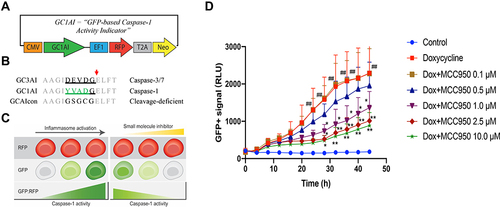
Figure 4 Identification of HEK293-NLRP3/CASP1-iASC-CASPorter clones by fluorescent microscope and flow cytometry. (A) Changes of GFP+ population after Dox 25 ng/mL treatment for 24h in HEK293-NLRP3/CASP1-iASC-CASPorter, HEK293-NLRP3/CASP1-iLacZ-CASPorter and HEK293-NLRP3/CASP1-iASC-Control under fluorescent microscope; (B) quantification of GFP+ population in the presence or absence of Dox for iASC-CASPorter, iLacZ-CASPorter and iASC-Control with flow cytometry. (C) statistical results of GFP+ population in iASC-CASPorter, iLacZ-CASPorter and iASC-Control with flow cytometry. (D) Protein expression (ASC, LacZ, Pro-caspase-1, Cleaved-caspase-1, NLRP3 and house keeping gene GAPDH) of iASC-CASPorter, iLacZ-CASPorter and iASC-Control with or without Dox 25 ng/mL treatment for 24h. Images are representative of three independent experiments. Values represent mean ± s.d. N3, NLRP3 C1, CASP1.
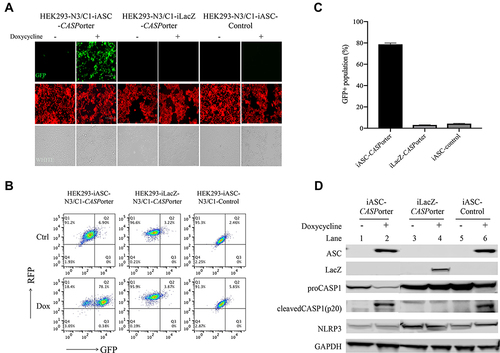
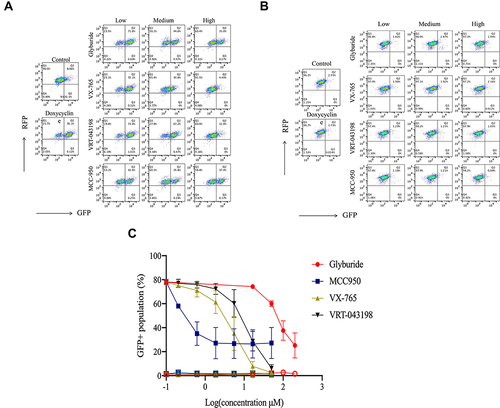
Figure 5 Effect of representative inhibitors of NLRP3 inflammasome on the fluorescent intensity in HEK293-NLRP3/CASP1-iASC-CASPorter and HEK293-NLRP3/CASP1-iLacZ-CASPorter. (A and B) The Dox-induced activation of NLRP3 inflammasome was dose-dependently inhibited by co-treatment with four reported inhibitors (MCC950 0–50 μM, Glyburide 0–200 μM, VX-765 0–50 μM, VRT-043198 0–50 μM) in HEK293-NLRP3/CASP1-iASC-CASPorter cells (A), but not in HEK293-NLRP3/CASP1-iLacZ-CASPorter cells (B). (C) Flow cytometric analysis of GFP+ population in these two cell models after co-treatment with Dox 25ng/mL and inhibitors (MCC950, Glyburide, VX-765, VRT-043198) for 24h. The solid and hollow symbols indicate HEK293-NLRP3/CASP1-iASC-CASPorter or -iLacZ cells, respectively. Values represent mean ± s.d.