Figures & data
Figure 1 Polyacrylamide gel electrophoresis of influenza A and H1N1 proteins. Lane 1, influenza A virus vaccine; lane 2, H1N1 virus vaccine; lane 3, broad weight molecular weight marker.
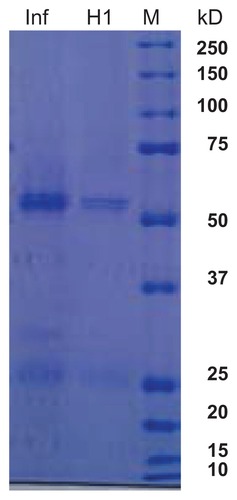
Table 1 Subject characteristics
Figure 2 Immunoblot analysis of IgG and IgE anti-H1N1 virus antibodies.
Abbreviation: Ig, immunoglobulin.

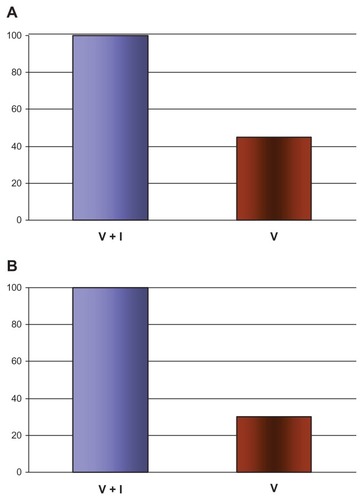
Figure 3 Comparison of IgG and IgE anti-H1N1 virus antibodies after vaccination with subsequent infection.
Abbreviation: Ig, immunoglobulin.