Figures & data
Table 1 Groups of Rats Used in the Present Study
Figure 1 Water avoidance stress protocol and cystometry setup. (A) Schematic drawing of the performing water avoidance stress to animals. (B) Schematic drawing of the cystometry setup. A pair of electrodes were embedded into the left abdominal external oblique muscle. A syringe pump is connected to a pressure transducer, which is connected to the intravesical catheter.
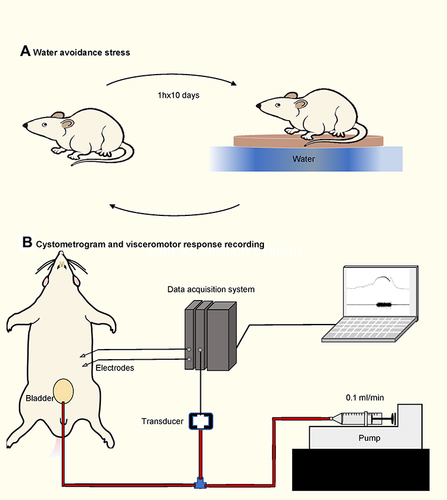
Table 2 Nucleotide Sequences of Primers for qRT-PCR
Figure 2 Cystometry and visceromotor response (VMR) recordings of first voiding cycle during saline infusion. (A) Representative recording examples of each group. (B) Alternations in pressure threshold. (C) Alternations in the ratio of VMR threshold/maximum intravesical pressure (IVPmax). (D) Alternations in IVPmax. (E) Alternations in bladder capacity. VT, VMR threshold. *Represents p<0.05. **Represents p<0.01.
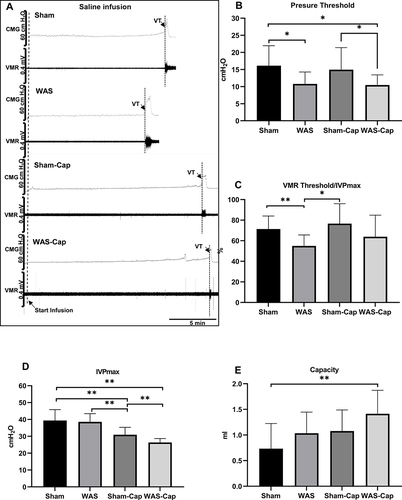
Figure 3 Cystometry and visceromotor response (VMR) recordings of continuous voiding cycles during saline infusion. (A) Representative recording examples of each group. (B) Alternations in pressure threshold. (C) Alternations in the ratio of VMR threshold/maximum intravesical pressure (IVPmax). (D) Alternations in IVPmax. (E) Alternations in the amplitude of VMR. (F) Alternations in the area under the curve (AUC) of VMR. (G) Alternations in the duration of VMR. VT, VMR threshold. *Represents p<0.05. **Represents p<0.01. ***Represents p<0.001. ****Represents p<0.0001.
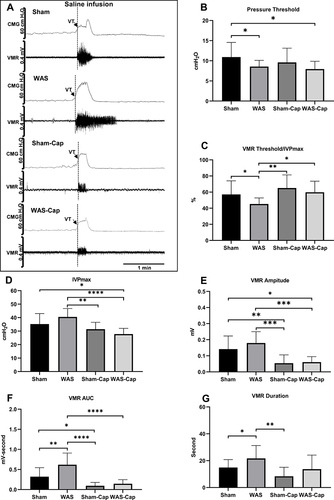
Figure 4 Cystometry and visceromotor response (VMR) recordings of first voiding cycle during endothelin-1 infusion. (A) Representative recording examples of each group. (B) Alternations in pressure threshold. (C) Alternations in the ratio of VMR threshold/maximum intravesical pressure (IVPmax). (D) Alternations in IVPmax. (E) Alternations in bladder capacity. VT, VMR threshold. * represents p<0.05. **Represents p<0.01. ***Represents p<0.001.
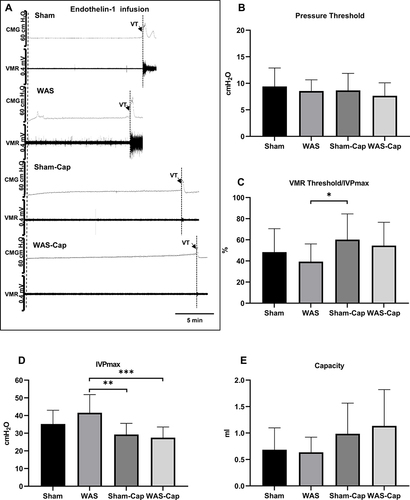
Figure 5 Cystometry and visceromotor response (VMR) recordings of continuous voiding cycles during saline infusion. (A) Representative recording examples of each group. (B) Alternations in pressure threshold. (C) Alternations in the ratio of VMR threshold/maximum intravesical pressure (IVPmax). (D) Alternations in IVPmax. (E) Alternations in the amplitude of VMR. (F) Alternations in the area under the curve (AUC) of VMR. (G) Alternations in the duration of VMR. VT, VMR threshold. *Represents p<0.05. **Represents p<0.01. ***Represents p<0.001. ****Represents p<0.0001.
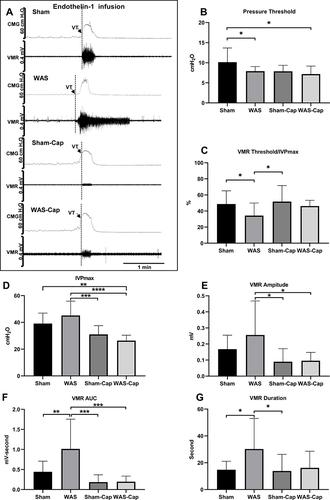
Figure 6 The expression of endothelin receptor type A (ETAR) in the bladder and L6-S1 dorsal root ganglions (DRGs). (A) Relative expression of ETAR protein in bladder tissue. (B) Relative expression of ETAR mRNA in bladder tissue. (C) Relative expression of ETAR mRNA in L6 DRGs. (D) Relative expression of ETAR mRNA in S1 DRGs. GAPDH, reduced glyceraldehyde-phosphate dehydrogenase.
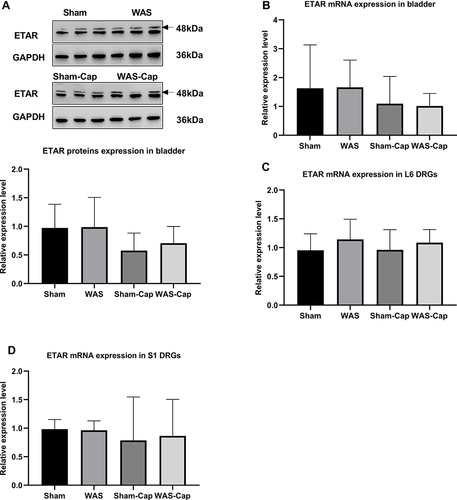
Figure 7 The toluidine blue staining and immunofluorescence staining of bladder and dorsal root ganglions (DRGs). (A–E) Representative toluidine blue staining of each group. Water avoidance appeared to show increased total mast cells, which could be attenuated by capsaicin pretreatment. (F–H) The immunoreactivity of endothelin receptor type A (ETAR, red) was positive and co-expressed with calcitonin gene-related peptides (CGRP, green) in the bladder mucosa of non-capsaicin-pretreated rats. (I–K) The immunoreactivity of ETAR was positive and co-expressed with CGRP) in the bladder muscle of non-capsaicin-pretreated rats. (L–N) The immunoreactivity of ETAR was positive and co-expressed with CGRP) in the L6 DRG of non-capsaicin-pretreated rats. Calibration bar was 20 μm and 5μm in histological staining and immunostaining, respectively. *Represents p<0.05.
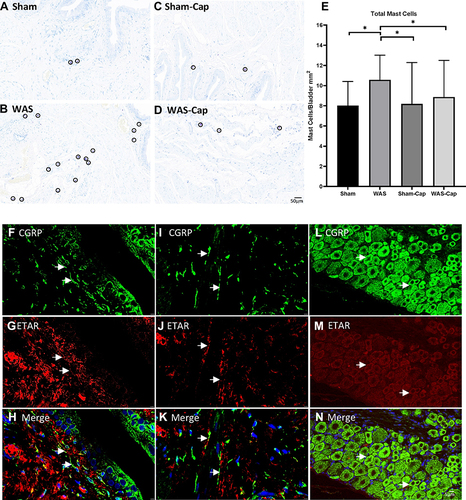
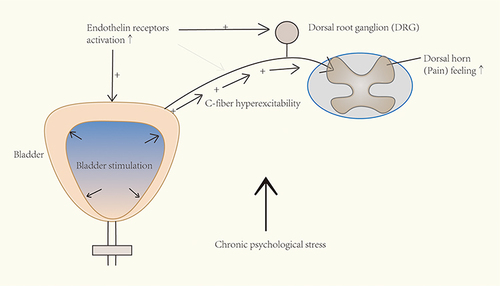
Figure 8 Schematic drawing of the involvement of endothelin pathway in chronic psychological stress-induced bladder hyperalgesia. After chronic stress exposure, the increased activation of endothelin receptors (possibly subtype A) may enhance the afferent input and in turn cause increased pain feeling.