Figures & data
Figure 2 The pathogenesis of IBD. Nod2 is highly expressed in Paneth cells and myeloid cells. Nod2 activated by muramyl dipeptide can activate the NF-κB pathway to produce α-defensins that directly regulate gut bacteria in Paneth cells. Nod2 signaling can drive the Th2-type immune response with the synergistic signals of OX40L and TSLPR. CARD9 is highly expressed in myeloid cells and can activate the NF-κB pathway to secrete pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-17A, IL-1β and IL-6. When CARD9 mutates to CARD9S12N, it can induce a Th2-type immune response. The IL-23 signaling pathway of Th17 cells is critical in the development of IBD. PTPN2 mediated interactions between intestinal epithelial cells and macrophages are necessary for maintaining the intestinal barrier function. The gut bacteria also affect the immune system by interacting with immune cells. DCs can induce Th0 cells to differentiate by producing different cytokines. Immune imbalance can cause excessive IL-13, IFN-γ and TNF-α to induce apoptosis of intestinal epithelial cells. Destruction of the intestinal barrier and invasion of the gut microbiota further leads to severe inflammation.
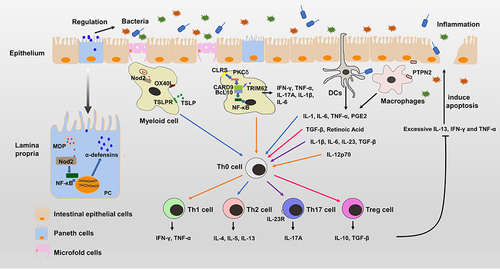
Figure 3 The pathogenesis of allergic asthma and the mechanisms of helminth therapy. When the immune system encounters allergens for the first time, airway epithelial cells activate ILC2s to induce a Th2-type immune response. Antigen presenting cells can also present allergens to Th0 cells and induce the differentiation of Th2 cells and Tfh cells. Tfh cells and IL-4 can induce B cells to produce allergen-specific IgE that binds to Fc receptors on mast cells, B cells and DCs. Eosinophils are recruited by IL-5 and release IFN-γ, which is involved in airway hyperresponsiveness. IL-17A and matrix metalloproteinase 9 also contribute to airway hyperresponsiveness. Upon subsequent exposure to allergens, immune cells can be quickly activated to cause inflammation. Helminths and helminth-derived molecules can induce the differentiation of Treg cells and anti-inflammatory cytokines in the parasitic location. Although some helminths are not exposed to lung tissue, the regulated immune cells that secret anti-inflammatory cytokines can migrate to inflammatory tissues to suppress the inflammation in lungs.
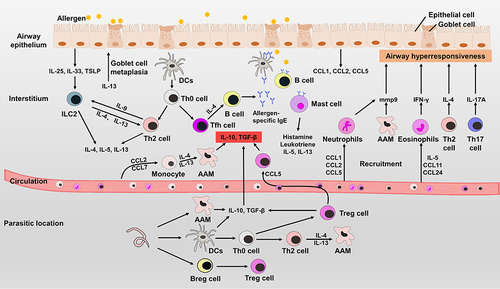
Table 1 Classification of Identified Helminth-Derived Molecules
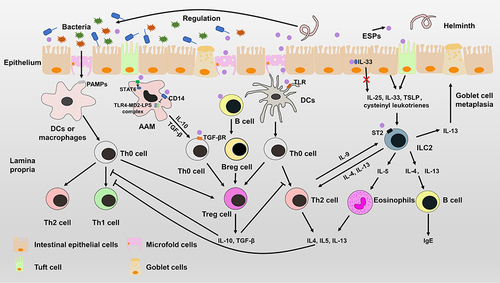
Figure 4 Immunomodulatory mechanisms of helminths. Helminths can induce intestinal epithelial cells to produce IL-25, IL-33 and TSLP, which can stimulate the proliferation of ILC2s. Activated ILC2s can secrete IL-4, IL-5 and IL-13 to expand Th2-type immune responses, which can suppress the Th1-type immune response. HPARI of Heligmosomoides polygyrus can bind to IL-33 and restrict it in necrotic cells. ESPs can induce the differentiation of Treg cells by interacting with antigen presenting cells or Th0 cells. ESPs not only induces alternative activation of macrophages (AAM) by phosphorylation of STAT6, but also inhibits the pro-inflammatory response mediated by TLR4-MD2-LPS complex. ESPs can affect the function of antigen-presenting cells to induce differentiation of Treg cells or Th2 cells. Treg cells, Breg cells and alternatively activated macrophages can secrete anti-inflammatory cytokines to suppress the Th1-type immune response and Th2-type immune response. In addition, helminths can regulate the diversity and composition of gut microbiota to alter the immune system.