Figures & data
Figure 1 Schematic depicting the activation and migration of resident and peripheral immune cells following SCI. After primary injury, resident astrocytes, microglia, and other glial cells are immediately activated and migrate to the injury site (top). Subsequently, peripheral inflammatory cells including neutrophils, bone marrow-derived macrophages, and lymphocytes infiltrate into the epicenter of the injured spinal cord, and these activated immune cells can exacerbate damage, causing a wider range of secondary injury. Glial cells (mainly astrocytes) form glial scar to seclude the damaged area, and microglia are mainly present around the injury site (middle). These persistent pathophysiological changes ultimately result in severe dysfunction below the damaged segment (bottom).
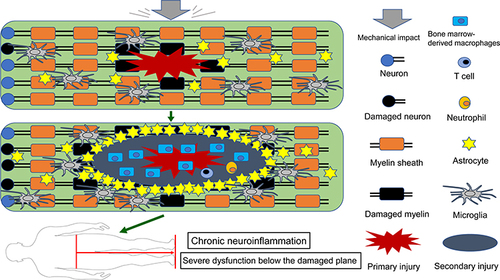
Table 1 The Immunomodulatory Mechanisms of Transplanted MSCs in Improving the Prognosis of SCI
Table 2 Completed Clinical Trials of MSCs in the Treatment of SCI
Figure 2 MSCs improve SCI prognosis via immunomodulatory effects. These transplanted MSCs inhibit an excessive inflammatory response by up-regulating anti-inflammatory immune cells and associated cytokines and down-regulating the pro-inflammatory immune cells and associated cytokines, thereby promoting anatomical repair and functional recovery.
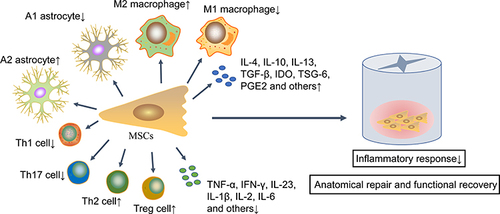
Figure 3 Preconditioning enhances the immunomodulatory ability and survival rate of MSCs in SCI. After SCI, the local harsh microenvironment causes a large amount of transplanted MSCs apoptosis. Various preconditioning strategies, including genetic modification, cytokines, hypoxia or other chemical molecules, can improve the immunomodulatory capacity, survival rate and homing ability of transplanted MSCs.
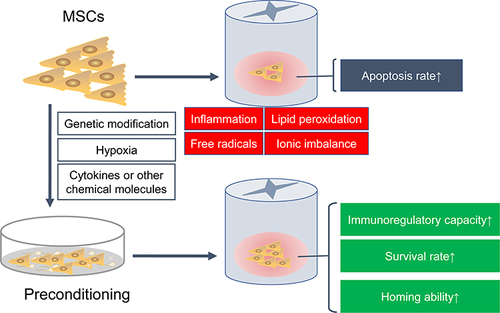
Table 3 The Immunosuppressive Effect of Pretreated MSCs and Extracellular Vesicles Secreted by MSCs in SCI
