Figures & data
Table 1 Studies Reporting Neuropsychiatric Diseases and Symptoms Related to COVID-19
Table 2 Evidence Supporting the Invasion of SARS-CoV-2 into the Central Nervous System
Figure 1 SARS-CoV-2 infection can cause brain damage through three possible routes. COVID-19 infection causes various brain injuries and diseases (eg, hypoxic brain injury, encephalitis, hemorrhagic necrotizing encephalopathy, acute disseminated encephalomyelitis, and acute stroke). SARS-CoV-2 invades the brain likely through three routes: (1) the vascular pathway where SARS-CoV-2 damages the blood-brain barrier to invade the brain via blood or lymphatic circulation; (2) the peripheral nerve pathway in which SARS-CoV-2 infects the olfactory bulb, the trigeminal nerve, the vagus nerve, and finally the brain through trans-neuronal or retrograde axonal transport; and (3) the cerebrospinal fluid pathway, SARS-CoV-2 can enter the brain through circulating cerebrospinal fluid to trigger a series of proinflammatory and immune responses that eventually result in various brain injuries with neuropsychiatric symptoms.
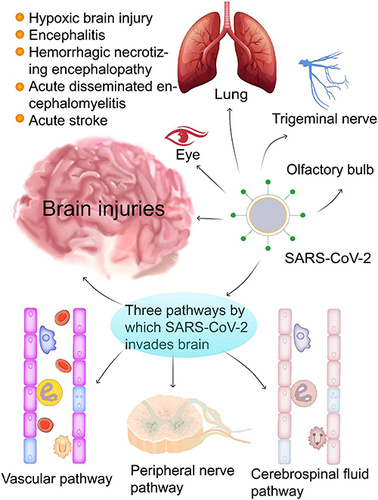
Figure 2 The potential role of ferroptosis in COVID-19-related brain injury. After SARS-CoV-2 infection, transferrin receptors recognize transferrin that carries Fe3+, which enters cells through endocytosis to form endosomes. IL-6 promotes ferritin synthesis, which stores Fe3+ and releases Fe3+ through ferritinophagy; after that, the endoplasmic reticulum metal reductase Steap3 reduces Fe3+ into Fe2+ to form LIP. GPX4 protects the cell against lipid peroxidation and inhibits ferroptosis. Under iron overload conditions combined with GPX4 depletion or inhibition, mitochondria generate large amounts of ROS, leading to lipid peroxidation, cell membrane damage, and ferroptosis. Excess ROS depletes intracellular GSH, and this depletion forms a positive feedback loop and aggravates lipid peroxidation. During this peroxidation, damage-associated molecular patterns and alarmins (eg, HMGB1, IL-33, TNF-α, IL-1 β, and IL-6) are released that activate NF-kB and other proinflammatory signaling pathways, eventually leading to neuroinflammation and cell death. SARS-CoV-2 infection can damage the brain, resulting in BBB disruption and bleeding accompanied by a cytokine storm.
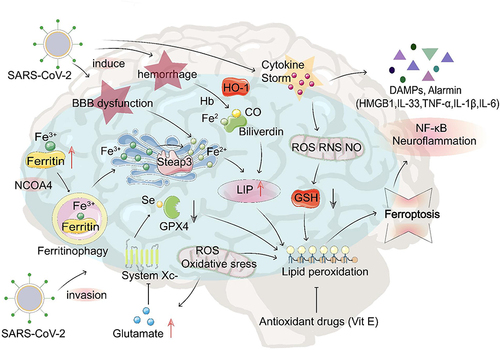
Table 3 Potential Therapeutic Targets for COVID-19-Related Brain Injury
