Figures & data
Table 1 Top 10 circRNAs That Most Likely Sponge miR-146a
Table 2 Antibodies Used for Western Blotting
Figure 1 Ischemia reperfusion (I/R) significantly downregulated the expression of miR-146a and upregulated the expression of circRNA Fbxl5. (A) Expression profile of miR-146a after I/R, with the lowest expression level occurring at 1 h after reperfusion; ****P<0.0001 compared with sham, n=6 per group. (B) CircRNA Fbxl5 was significantly upregulated at 1 h after reperfusion; ****P<0.0001 compared with sham, n=6 per group. (C) The expression variation of circRNA Fbxl5 in neonatal mice ventricular myocytes (NMVMs) and cardiac fibroblasts (CFs) between normoxia and anoxia reoxygenation (A/R); *P<0.05 compared with control group (Ctrl), n=5 per group. (D) Sanger sequencing of the amplified products indicated that circRNA Fbxl5 was produced from the Fbxl5 gene and demonstrated the head-to-tail splicing of the latter half of exon 4 (100nt). (E) Divergent primers amplify circRNAs in cDNA but not genomic DNA (gDNA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
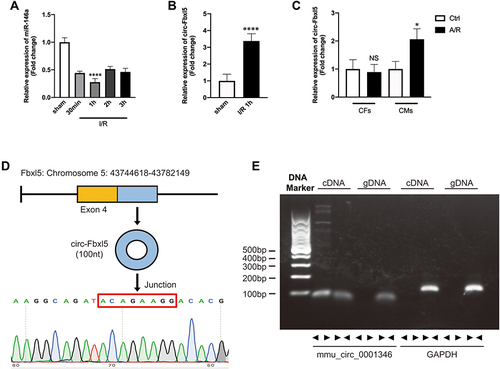
Figure 2 CircRNA Fbxl5 sponges miR-146a. (A) Graphical illustration showing the predicted sites of miR-146a for binding to circRNA Fbxl5 and the corresponding mutation. (B) Dual luciferase reporter assay showing the interaction of miR-146a and circRNA Fbxl5; ***P<0.001 compared with miR-NC, n=3 per group. (C and D) AGO2 protein-based RNA immunoprecipitation assay for the interaction between miR-146a and circRNA Fbxl5; ***P<0.001 compared with IgG, n=3 per group.
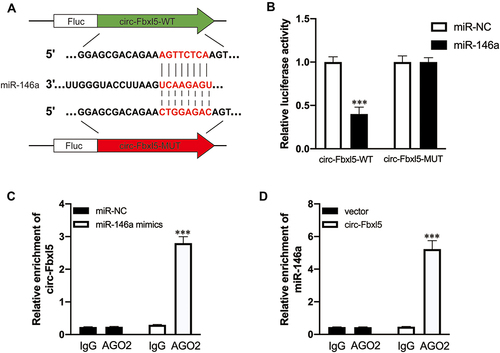
Figure 3 CircRNA Fbxl5 promotes the deterioration of cardiac function after myocardial ischemia reperfusion (MI/R). (A) The timeline of the in vivo experiment. (B) TUNEL-based evaluation of apoptosis in the ventricular myocardium; TUNEL positive cardiomyocytes were presented in green while nuclei were presented in blue by DAPI. (C–E) Representative Evans blue/TTC staining of the cross section of the ventricles (C) and quantitative analysis of the infarct size (Inf) between groups (D–E), showing that knockdown of circRNA Fbxl5 significantly reduced the infarct size after MI/R; ****P<0.0001 compared with vehicle, n=6 per group. (F–I) Representative Western blots (F) and quantitative analysis of Bcl-2, Bax and cleaved caspase 3 proteins between groups (G–I), showing that knockdown of circRNA Fbxl5 significantly attenuated cardiomyocyte apoptosis after MI/R; ***P<0.001 and ****P<0.0001 compared with sham; ##P<0.01, ###P<0.001 and ####P<0.0001 compared with vehicle; NS denotes non-significance compared with vehicle, n=3 per group. (J–L) Representative echocardiography (J) and quantitative analysis of left ventricular ejection fraction (LVEF) and fractional shortening (FS) between groups (K–L), showing that knockdown of circRNA Fbxl5 significantly improved cardiac function after MI/R; *P<0.05 compared with vehicle; NS denotes not significance compared with vehicle, n=3 per group.
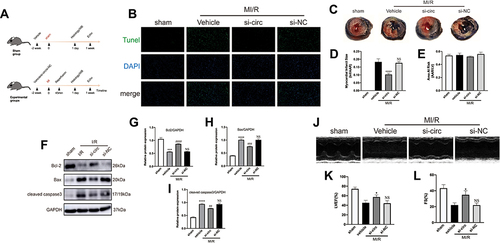
Figure 4 CircRNA Fbxl5 aggravates cardiomyocyte apoptosis after anoxia reoxygenation (A/R). (A–D) Representative Western blots (A) and quantitative analysis of Bcl-2, Bax and cleaved caspase 3 proteins between groups (B–D), showing that knockdown of circRNA Fbxl5 significantly attenuated cardiomyocyte apoptosis after A/R; *P<0.05, **P<0.01 and ***P<0.001 compared with A/R; NS denotes non-significance compared with A/R. (E) Representative TUNEL assay of apoptosis of cardiomyocytes between groups, showing that knockdown of circRNA Fbxl5 significantly attenuated cardiomyocyte apoptosis after A/R.
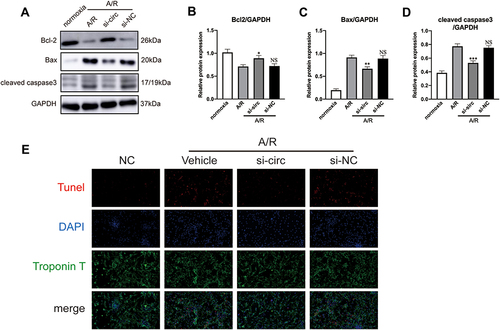
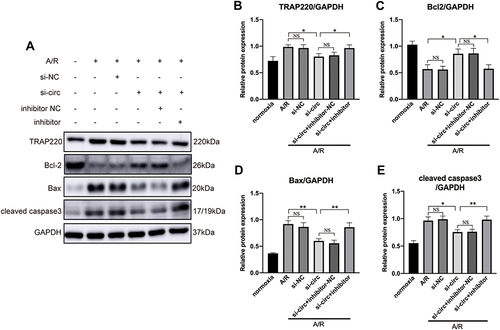
Figure 5 Representative Western blots (A) and quantitative analysis (B–E) of TRAP220 (MED1), Bcl2, Bax and cleaved caspase 3, showing that inhibition of miR-146a reversed the effect of circRNA Fbxl5 knockdown on the expression of MED1 and cardiomyocyte apoptosis; *P<0.05 and **P<0.01; NS=non-significance.