Figures & data
Figure 1 Trained immunity induced by BCG+BLP protects neonatal mice against microbial sepsis. Neonatal mice were injected with BCG, BLP, or BCG+BLP and rested for 3 days to induce trained immunity, and then challenged with cecal slurry peritonitis-induced polymicrobial sepsis. (A and B) Substantially improved survival in BCG+BLP-treated mice subjected to either mid-grade sepsis (p=0.0011) (A) or high-grade sepsis (p=0.002) (B) (n=18 per group) compared to PBS-treated mice (n=17). (C) Data shown are the results of serum TNF-α and IL-6 levels at 0, 2, and 6 h post septic challenge. (D) Bacterial counts in the blood, liver, spleen, and lungs assessed at 12 and 24 h post septic challenge. (E) Data shown are subpopulations (%) of PMNs (CD11b+F4/80−Gr1hi) and macrophages (CD11b+F4/80+CD11clo) in the peritoneal lavage collected at 0, 6, and 12 h post septic challenge. Data in (C–E) are mean ± SD (4–5 mice per time point) and representative of three independent experiments. *p<0.05, **p<0.01 versus PBS-treated mice; ≠p<0.05, ≠≠p<0.01 versus BCG- or BLP-treated mice.
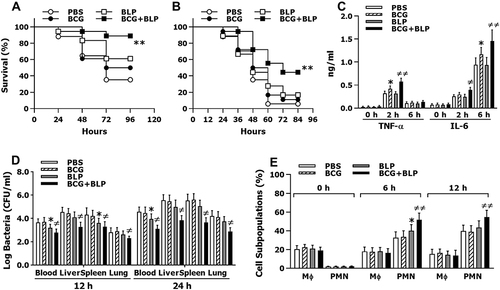
Figure 2 Training innate immunity by BCG+BLP enhances nuclear transactivation of NF-κB p65 and boosts inflammatory cytokine and chemokine release. Isolated neonatal murine peritoneal macrophages or BMMs were stimulated with BCG, BLP, or BCG+BLP for 24 h, rested for additional 3 days, and further challenged with heat-killed bacteria. (A) TNF-α, IL-6, IL-12p70, and CXCL2 in the supernatants were measured 18 h post bacterial challenge. (B) The binding of p65 to TNF-α, IL-6, IL-12p70, and CXCL2 promoters were measured 1 h post bacterial challenge. Data are mean ± SD from four to six separate experiments. *p<0.05, **p<0.01 versus PBS-incubated macrophages; ≠≠p<0.01 versus BCG- or BLP-stimulated macrophages.
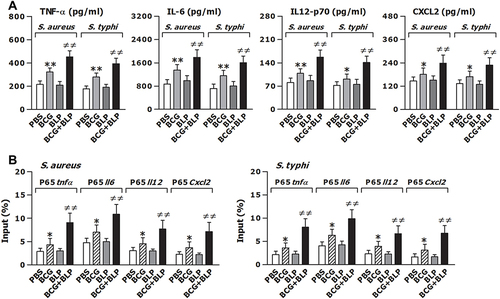
Figure 3 Training innate immunity by BCG+BLP accelerates phagosome maturation and augments phagocyte-associated bactericidal activity. Isolated neonatal murine peritoneal macrophages were stimulated with BCG, BLP, or BCG+BLP for 24 h and rested for additional 3 days. (A) After being incubated with heat-killed, FITC-conjugated S. aureus or S. typhi for 60 min, bacterial phagocytosis was assessed in neonatal macrophages. (B) After being challenged with live S. aureus or S. typhi for 2 h, intracellular killing of the ingested bacteria was assessed in neonatal macrophages. (C) After being incubated with fluorescent probe-labeled S. aureus or S. typhi for different time points, phagosomal pH was assessed in neonatal macrophages. (D) After being incubated with heat-killed S. aureus or S. typhi for 30, 60, and 90 min, phagolysosome fusion was assessed in neonatal macrophages. Data are mean ± SD from five to six separate experiments in duplicate or triplicate. *p<0.05 versus PBS-incubated macrophages; ≠p<0.05, ≠≠p<0.01 versus BCG- or BLP-stimulated macrophages.
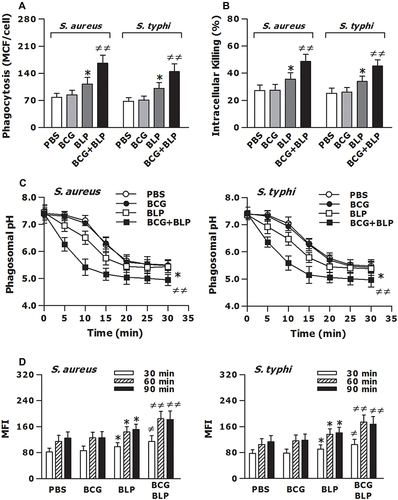
Figure 4 Training innate immunity by BCG+BLP induces epigenetic reprogramming with histone modifications at the promoters of TNF-α, IL-6, Acp5, and Camp. Isolated neonatal murine BMMs were stimulated with BCG, BLP, or BCG+BLP for 24 h and rested for additional 3 days. H3K4me3 (A and B), H3K27Ac (C and D), and H3K9me3 (E and F) at the promoters of inflammatory response genes TNF-α and IL-6 (A, C, E) and antimicrobial effector genes Acp5 and Camp (B, D, F) were assessed in neonatal macrophages. Data are mean ± SD from five separate experiments in duplicate. *p<0.05, **p<0.01 versus PBS-incubated macrophages; ≠≠p<0.01 versus BCG- or BLP-stimulated macrophages.
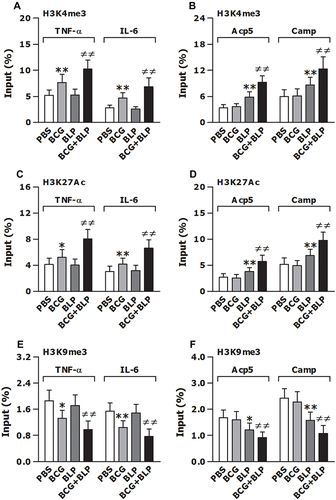
Figure 5 Blockage of histone modifications abrogates BCG+BLP-boosted inflammatory response and bactericidal activity. Isolated neonatal murine peritoneal macrophages or BMMs were pretreated with DMSO, UNC1999 (UNC), C646, or UNC1999+C646 (Inhibitors) for 1 h before training of innate immunity. (A) H3K4me3 and H3K27Ac at the promoters of TNF-α, IL-6, Acp5, and Camp were assessed in neonatal macrophages before bacterial challenge. (B) TNF-α, IL-6, IL-12p70, and CXCL2 in the supernatants were measured 18 h post bacterial challenge. (C) Bacterial phagocytosis and intracellular killing were assessed in neonatal macrophages 60 min and 2 h after being incubated with FITC-conjugated or live S. aureus. Phagosomal pH and phagolysosome fusion were assessed in neonatal macrophages after being incubated with fluorescent probe-labeled or heat-killed S. aureus. Data are mean ± SD from five to six separate experiments in duplicate or triplicate. **p<0.01 versus DMSO-pretreated, PBS-incubated macrophages; ≠p<0.05, ≠≠p<0.01 versus DMSO-pretreated, BCG+BLP-stimulated macrophages.
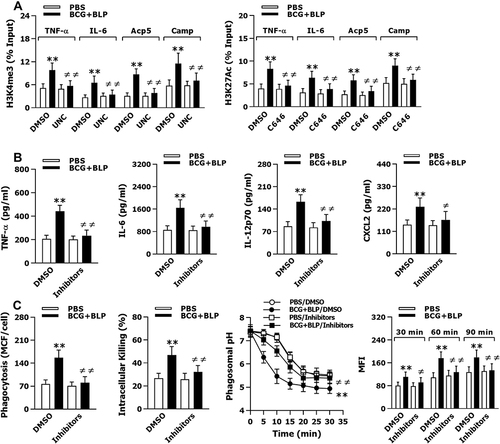
Figure 6 Training innate immunity by BCG+BLP leads to altered intracellular metabolic pathways. Isolated neonatal murine peritoneal macrophages were stimulated with BCG, BLP, or BCG+BLP for 24 h and rested for additional 3 days. After being incubated with heat-killed S. aureus or S. typhi for 6 h, glucose consumption (A) and lactate secretion (B) were assessed in neonatal macrophages. Basal and max extracellular acidification rate (ECAR) (C) and oxygen consumption rate (OCR) (D) were measured in neonatal macrophages before bacterial challenge. Glutamate (E) and acetyl-CoA (F) were assessed in neonatal macrophages after being incubated with heat-killed S. aureus or S. typhi for 6 h. Data are mean ± SD from four to five separate experiments in duplicate or triplicate. *p<0.05 versus PBS-incubated macrophages; ≠≠p<0.01 versus BCG- or BLP-stimulated macrophages.
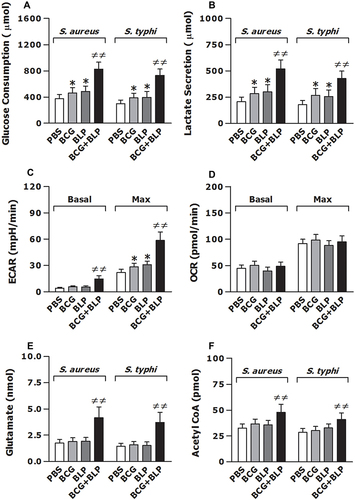
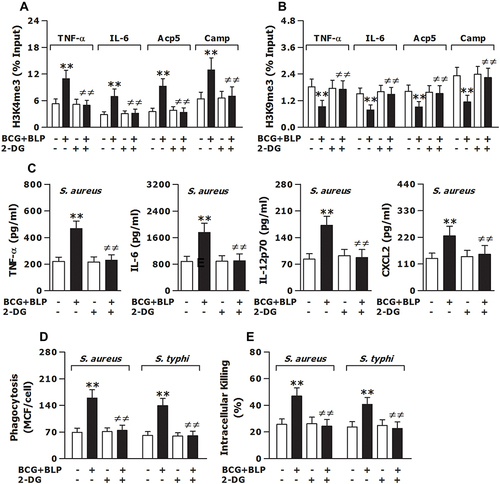
Figure 7 Inhibition of glycolysis prevents BCG+BLP-induced trained immunity with disrupted epigenetic reprogramming and abrogated augmentations in both inflammatory and antimicrobial responses. Isolated neonatal murine peritoneal macrophages or BMMs were pretreated with culture medium (CM) or 2-DG for 1 h before training of innate immunity. (A and B) H3K4me3 and H3K9me3 at the promoters of TNF-α, IL-6, Acp5, and Camp were assessed in neonatal macrophages before bacterial challenge. (C) TNF-α, IL-6, IL-12p70, and CXCL2 in the supernatants were measured 18 h post bacterial challenge. (D and E) Bacterial phagocytosis and intracellular killing were assessed in neonatal macrophages 60 min and 2 h after being incubated with FITC-conjugated or live S. aureus or S. typhi. Data are mean ± SD from five to six separate experiments in duplicate or triplicate. **p<0.01 versus CM-pretreated, PBS-incubated macrophages; ≠≠p<0.01 versus CM-pretreated, BCG+BLP-stimulated macrophages.