Figures & data
Table 1 Characteristics of Sarcoidosis Patients and Control Subjects
Table 2 Antibodies Used for Flow Cytometry Analysis
Figure 1 CHIT1 activity and concentration in sarcoidosis patients’ serum is highly upregulated. (A) Chitinolytic activity in serum and (B) CHIT1 concentration from control donors and sarcoidosis patients. Data presented as box and whiskers plot **** P<0.0001. (C) The correlation of the chitinolytic activity and CHIT1 concentration in serum of patients with sarcoidosis. Correlation coefficient r = 0.8721, p<0.000001.
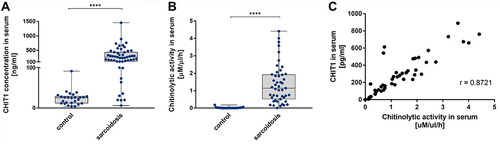
Figure 2 Pathological macrophages are a source of CHIT1 in sarcoidosis. (A) Representative immunocytochemistry for CHIT1 in BALF cells smears from sarcoidosis patients (n = 12); (B and C) Representative images for CHIT1 and AMCase immunohistochemistry of the bronchial mucosa and mediastinal lymph nodes specimens of sarcoidosis patients (n = 15).
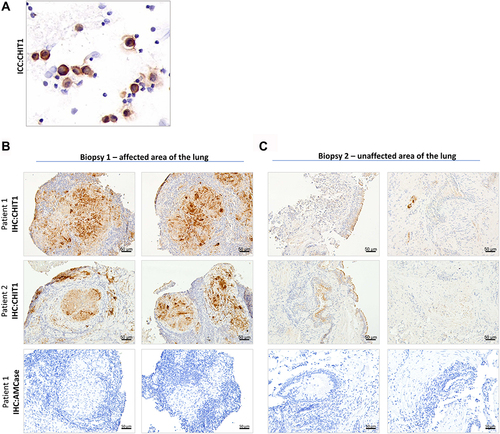
Figure 3 OATD-01 inhibits upregulated chitinolytic activity in serum from sarcoidosis patients and has a direct effect on patients’ BALF macrophages. (A) Ex vivo inhibition of the chitinolytic activity in sarcoidosis patients serum samples (n = 2) by OATD-01. (B) Ex vivo inhibition of the chitinolytic activity in sarcoidosis patients BALF samples (n = 2) by OATD-01. (C–H) The levels of immune responses’ modulators (CCL4, IL-15, PDGFβ, IL-1 receptor antagonist, IL-6, IL-17) in sarcoidosis BALF macrophages supernatants (n = 6) after OATD-01 treatment. The concentration of analytes in supernatants from untreated and OATD-01-treated macrophages were compared using two-tailed paired t-test. *P<0.05, **P<0.01.
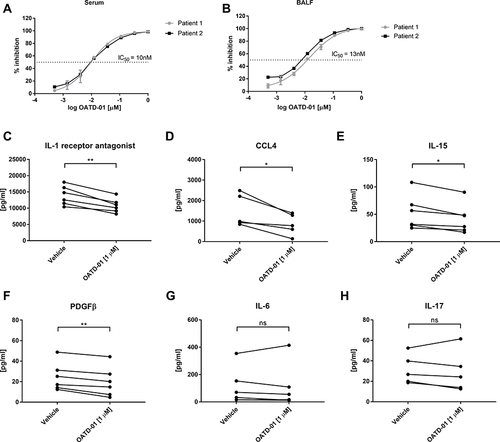
Figure 4 OATD-01 shows the anti-inflammatory effects in 10-day-long MWCNT + ESAT-6 model of granulomatous inflammation in mice. (A) A scheme of the in vivo experiment. MWCNT + ESAT-6 was administered oropharyngeally on 3 consecutive days and OATD-01 was administered orally, once a day for 10 days at 100 m/kg dose. (B) Analysis of CCL2 levels in murine BALF following OATD-01 administration in comparison to the vehicle-treated animals. (C) Analysis of CCL4 levels in murine BALF following OATD-01 administration in comparison to the vehicle-treated animals. (D) Chitinolytic activity in BALF following OATD-01 administration as compared to the vehicle-treated animals. (E–G) Flow cytometry analysis of BALF leukocyte subpopulations such as alveolar macrophages and neutrophils following 10-day treatment with OATD-01 as compared to the vehicle-treated mice. Data presented as mean ± SEM; *p<0.05, **p<0.01, ***P<0.001, ****P<0.0001.
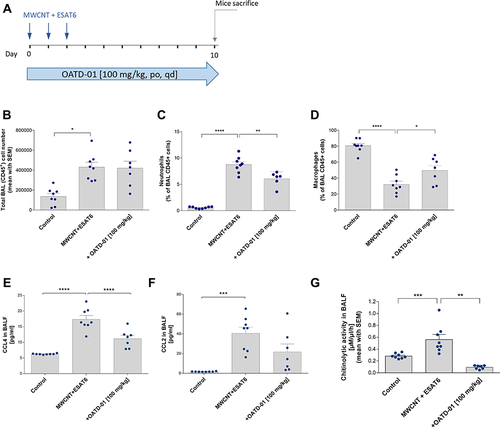
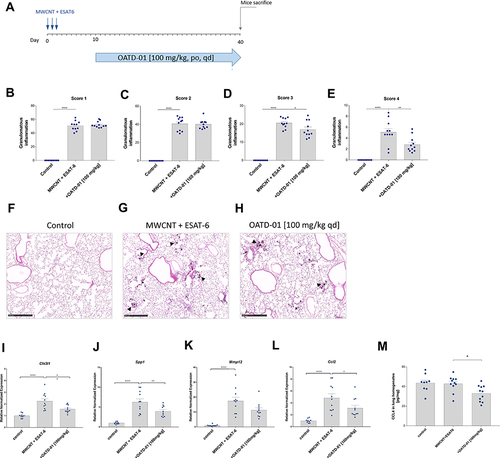
Figure 5 OATD-01 demonstrates therapeutic effects in 40-day-long MWCNT + ESAT-6 murine model of granulomatous inflammation affecting granuloma maturation and pro-inflammatory mediators expression. (A) A scheme of the in vivo experiment. MWCNT + ESAT-6 was administered oropharyngeally on 3 consecutive days and OATD-01 was dosed in a therapeutic treatment regimen, once a day, for 30 days at 100 mg/kg dose. (B–E) Analysis of pulmonary granulomatous inflammation by histological assessment in the lung sections (n = 3 per animal) in animals following OATD-01 administration in comparison to the vehicle-treated control group. (F–H) Representative images of lung granulomatous changes visualized by the HE staining in control animals, mice instilled with MWCNT + ESAT-6 only or dosed also with OATD-01. The arrows indicate granuloma-like changes. (I–L) The relative expression of pro-inflammatory mediators (Chi3l1, Spp1, Mmp12, Ccl2) in the lungs in vehicle- and OATD-01-treated animals evaluated by qtPCR. (M) Analysis of CCL4 levels in lung homogenate following OATD-01 treatment in comparison to the vehicle-treated animals. Data presented as mean ± SEM; *p<0.05, **p<0.01, ***P<0.001, ****P<0.0001.