Figures & data
Table 1 Clinical Characteristics of five Patients with Severe CVT During Admission
Table 2 PC/PS Levels and Sequence Map in Patients and Their Families
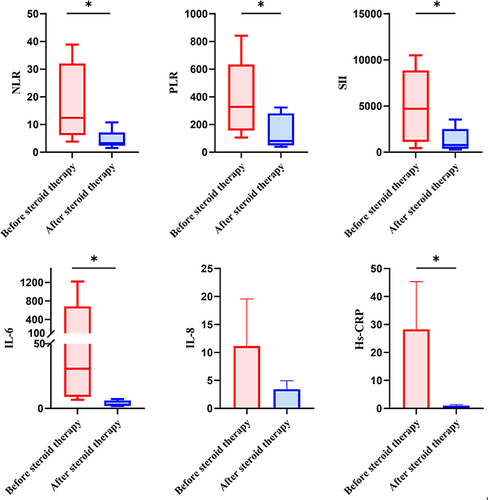
Table 3 Levels of Inflammatory Indexes in CSF Before and After Steroid Therapy
Figure 2 Changes in neurological impairment and intracranial pressure at discharge and 6-month follow-up. (*P<0.05).
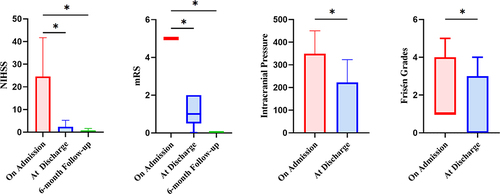
Figure 3 Case 2: (A) Brain CT on admission indicated new cerebral hemorrhagic infarction in the right frontoparietotemporal lobe with obvious local edema and right cerebral hernia. (B) MRBTI on admission displayed superior sagittal sinus thrombosis. (C) After methylprednisolone therapy, compared with A, brain CT demonstrated that the edema and hemorrhage area of the right frontoparietotemporal lobe were significantly reduced and the left shift of midline structure alleviated. (D) After methylprednisolone therapy, compared with (B), MRBTI showed the thrombus in superior sagittal sinus was reduced. (E) At 3-month follow-up, compared with (C), brain CT showed the edema of the right frontoparietotemporal lobe disappeared and only a few previous infarcts remained. (F) At 3-month follow-up, CE-MRV indicated a considerable decrease of thrombus in the superior sagittal sinus without new thrombosis in comparison to (D). (G) At 6-month follow-up, compared with (E), Axial T2-MRI revealed no significant changes in right frontoparietotemporal lobe and no new infarcts were detected. (H) At 6-month follow-up, MRBTI demonstrated a further reduction of superior sagittal sinus thrombus compared to (F), leaving a negligible quantity of residual thrombus.
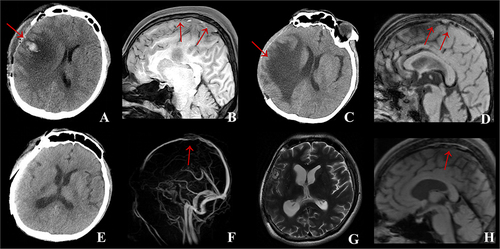
Figure 4 Case 4: (A) Brain CT on admission showed the thrombosis of straight sinus and left temporal-occipital lobe cerebral infarction with hemorrhage. (B) CE-MRBTI on admission revealed thrombosis occurred in the vein of Galen, straight sinus, superior sagittal sinus and torcular herophili. (C) CE-MRV on admission displayed that thrombosis was involved in the superior sagittal sinus, torcular herophili, left transverse sinus, sigmoid sinus and internal jugular vein. (D) After methylprednisolone therapy, brain CT demonstrated the straight sinus thrombosis and left temporaloccipital lobe cerebral infarction with hemorrhage disappeared. (E) After methylprednisolone therapy, MRBTI showed a reduction of thrombi in the vein of Galen, straight sinus, superior sagittal sinus and torcular herophili. (F) After methylprednisolone therapy, compared with (C), CE-MRV revealed a considerable decrease of thrombi in the superior sagittal sinus, torcular herophili, left transverse sinus, sigmoid sinus and internal jugular vein. (G) At 6-month follow-up, compared with (E), MRBTI showed thrombi in the vein of Galen, straight sinus and torcular herophili disappeared. (H) At 6-month follow-up, compared with (F), CE-MRV indicated thrombi of left transverse sinus and sigmoid sinus reduced.
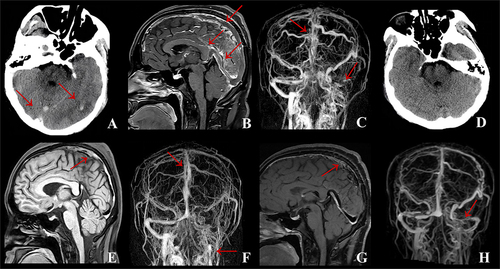
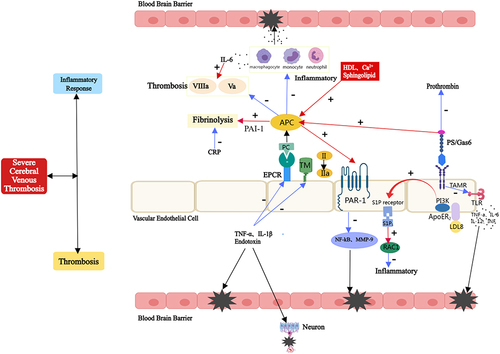
Figure 5 Anticoagulant and anti-inflammatory effects of PC/PS and the pathophysiology of hereditary PCD/PSD in severe CVT. PC interacts with the thrombin-TM complex and the EPCR to activate APC, which inhibits the anticoagulant effects of factors Va and VIIIa. PS, sphingolipids, and HDL can all accelerate this process. Furthermore, APC can promote fibrinolysis by inhibiting PAI-1. PS, in addition to being a cofactor of APC, can directly inhibit prothrombin, resulting in an anticoagulant effect. APC can activate PAR-1 in an EPCR-dependent manner, inhibit the transcriptional activation of MMP-9 dependent on NF- κB, and then protect the BBB. In addition, it exerts anti-inflammatory effects through S1P and Rac1-dependent mechanisms. PS inhibits the TLR -induced signaling cascade and inhibits inflammatory cytokines by binding to TAMRs. CRP can promote the formation of PAI-1 and tissue factor, and inhibit fibrinolysis. Endotoxin, IL-1β and TNF-α reduce APC production by down-regulating thrombomodulin and EPCR, thereby inhibiting anticoagulant and anti-inflammatory responses. Consequently, for hereditary PCD/PSD, the inflammatory and thrombosis cascade continues to amplify, resulting in damage to the BBB and nerve cells, thus aggravating the patient’s condition. Blue Arrow: the inhibiting effect, Red Arrow: the activation effect, Black Star and Arrow: the damaging effect.