Figures & data
Table 1 Overview of randomized, double-blind clinical trials of diclofenac sodium topical solution
Figure 1 Improvement from baseline to final assessment in WOMAC pain score with diclofenac sodium topical solution and vehicle control. Pain score was based on five questions scored on a scale of 0 to 4.
Abbreviation: WOMAC, Western Ontario and McMaster Universities.
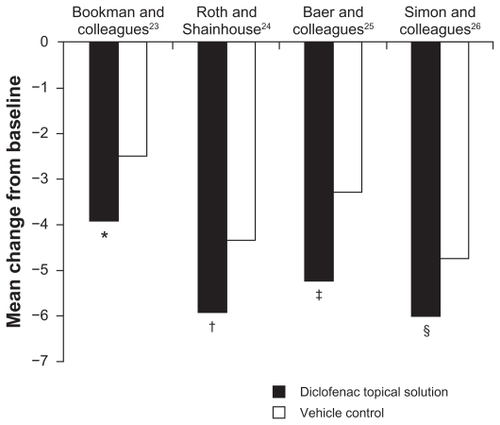
Figure 2 Improvement from baseline to final assessment in WOMAC physical function score with diclofenac sodium topical solution and vehicle control. Physical function score was based on 17 questions scored on a scale of 0 to 4.
Abbreviation: WOMAC, Western Ontario and McMaster Universities.
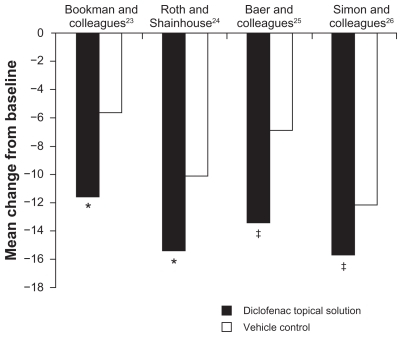
Table 2 Patient perception of osteoarthritis symptoms and overall health status as assessed by the PGA or POHA, after treatment with diclofenac sodium topical solution, vehicle control, or placebo
Figure 3 Percent change from baseline to final assessment in efficacy variables with diclofenac sodium topical solution and oral diclofenac.
Abbreviations: PGA, Patient Global Assessment; POHA, Patient Overall Health Assessment.
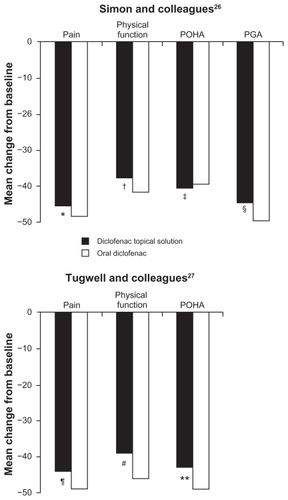
Table 3 Adverse events in studies comparing diclofenac sodium topical solution with oral diclofenacCitation26,Citation27
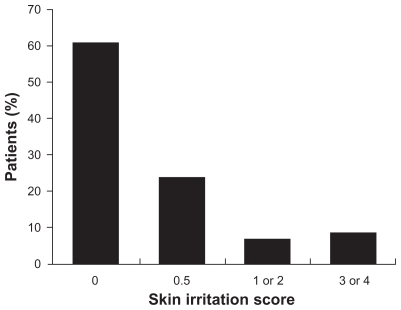
Figure 4 Skin irritation scores in a long-term, open-label study of diclofenac sodium topical solution.Citation36