Figures & data
Figure 1 Inclusion criteria. Shown are the inclusion criteria for the retrospective cohort study. A total of 299 patients were assessed for study eligibility, of which 225 were eligible and included for review. Note that patients who received treatment prior to the first tumor board were excluded from the study.
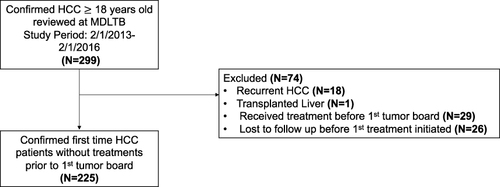
Figure 2 Flowcharts demonstrating MDLTB recommendation compliance to guidelines and adherence of physician treatment to MDLTB recommendation. (A) Flow chart demonstrating compliance and adherence according to the contemporaneous (2012) guideline. 94.7% of MDLTB recommendations followed the concurrent 2012 BCLC classification guideline. Treatment compliance stratified by MDLTB adherence is shown above. Overall, 85.3% of MDLTB treatment recommendations were adhered to during treatment. (B) Changes in guideline compliance between 2012 and 2022 guidelines. Note that all patients in the study were treated prior to the publication of the 2022 guideline. One Stage 0 and one Stage B patient who received treatment non-compliant with the 2012 guideline are compliant with the 2022 guideline. Ten Stage A patients’ treatments which were compliant with the 2012 guideline are not compliant with the 2022 guideline. There are no changes in treatment compliance in the Stage C and Stage D patients.
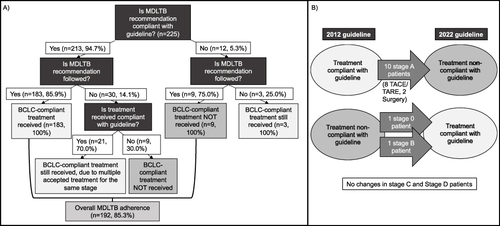
Table 1 Survival Time for Each Treatment Type Stratified by Disease Stage
Table 2 Survival Models Comparing Curative and Palliative Treatment in Patients with Unifocal Stage A Disease Controlling for Potential Confounding Factors
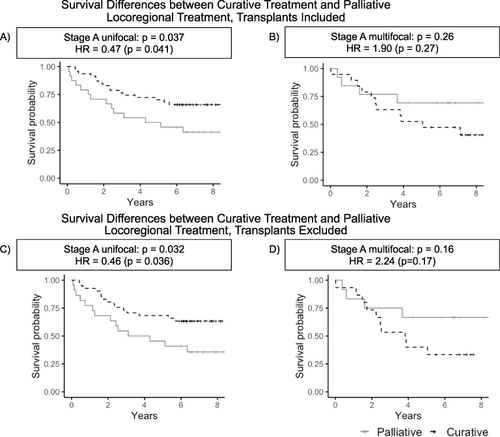
Figure 3 Survival differences between curative treatment and palliative locoregional treatment, with and without transplants. Shown are survival curves for patients with Stage A disease who received curative treatment and for patients who received palliative locoregional treatment, stratified by unifocal (A) vs multifocal disease (B). To control for liver transplant as a confounder, the analysis was repeated in a subset of the cohort excluding transplants (C and D). Stage A unifocal patients who received curative treatment had statistically significant improved survival compared to Stage A unifocal patients who received palliative locoregional therapy (p=0.041, log-rank test; see (A)); this survival benefit is also present in the subset excluding transplants (p=0.036, see (C)). Such a survival benefit is not present in patients with Stage A multifocal HCC (p=0.27, see (B)), and is also absent in the subset excluding liver transplants (p=0.17, see (D)).