Figures & data
Figure 1 Effect of topically applied mepivacaine on mechanical hyperalgesia in rats with gp120-induced neuropathic pain.
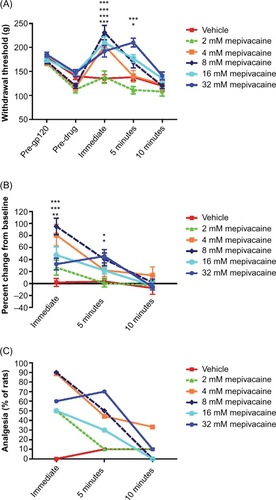
Figure 2 Effect of topically applied mepivacaine on thermal hyperalgesia in rats with gp120-induced neuropathic pain.
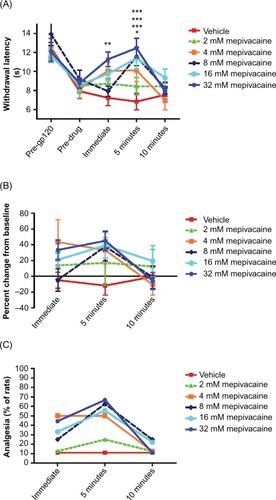
Figure 3 Effect of topically applied lidocaine on mechanical hyperalgesia in rats with gp120-induced neuropathic pain.
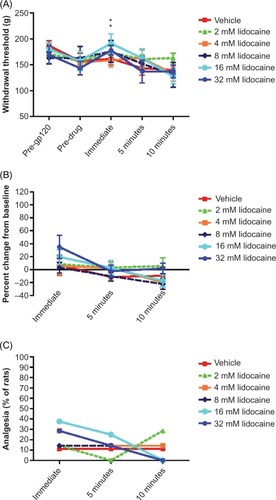
Figure 4 Effect of topically applied lidocaine on thermal hyperalgesia in rats with gp120-induced neuropathic pain.
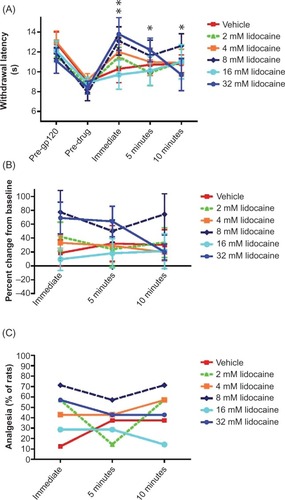
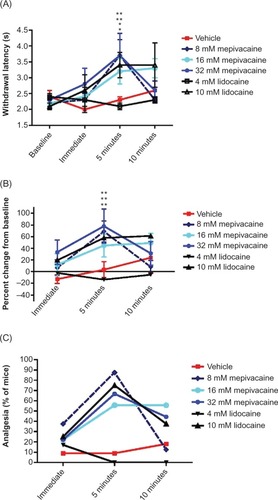
Figure 5 Effects of topically applied mepivacaine and lidocaine on acute nociception in the mouse tail flick assay.