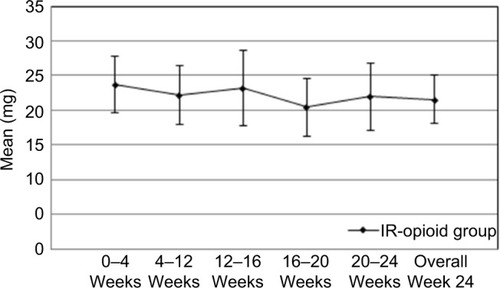
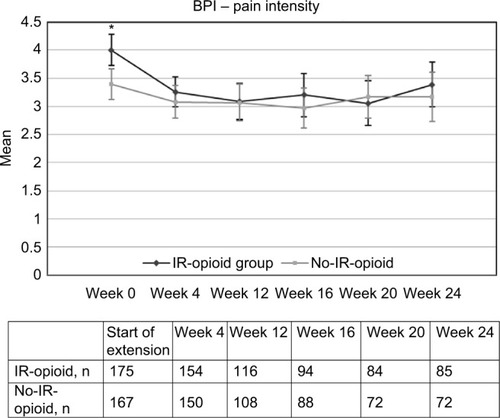
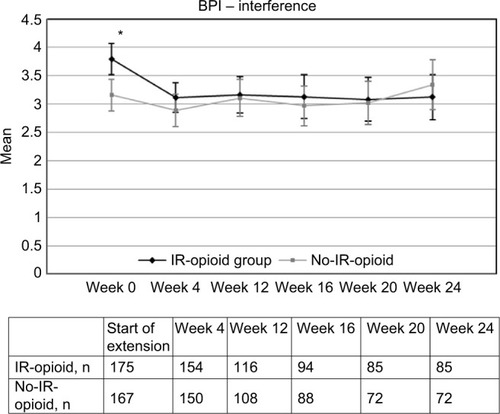
Figures & data
Table 1 Comparative characteristics, enrolled extension-trial population
Table 2 Incidence of TEAEs (≥5%) during BTDS treatment for moderate–severe chronic pain: IR-opioid group vs no-IR-opioid group
Table 3 Published studies where IR opioids were used as supplemental analgesia during transdermal buprenorphine therapy for chronic pain