Figures & data
Table 1 Demographic and clinical characteristics of patients with CLBP enrolled in 4 randomized, placebo-controlled trials of duloxetine treatmentCitation14–Citation17
Table 2 Disposition of patients with CLBP enrolled in 4 randomized, placebo-controlled trials of duloxetine treatmentCitation14–Citation17
Figure 1 Proportion of patients with CLBP who achieved ≥30% or ≥50% reduction in BPI Severity average pain after 12–14 weeks treatment with duloxetine 60 mg (black bars; n = 642) or placebo (white bars; n = 653).
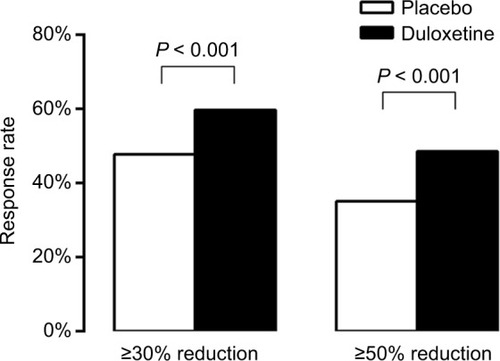
Figure 2 (A, B) Responder analysis (A, ≥30% reduction in BPI average pain; B, ≥50% reduction in BPI average pain) for patients with CLBP treated with duloxetine 60 mg for 12–14 weeks.
Abbreviations: BPI, Brief Pain Inventory; CI, confidence interval; CLBP, chronic low back pain; F, female; M, male; MBM, Michigan Body Map; RR, relative risk; y, years.
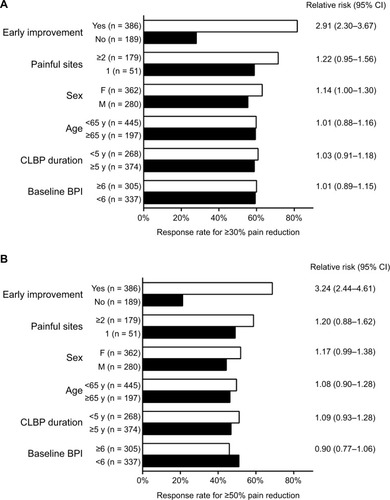
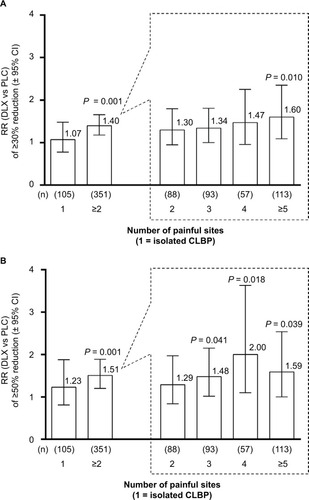
Figure 3 (A, B) Effect of the number of painful body sites (according to the MBM) on the RR (duloxetine 60 mg vs placebo) of achieving ≥30% reduction (A) or ≥50% reduction (B) in BPI average pain in patients with 1 (isolated CLBP), ≥2, 2, 3, 4, or ≥5 painful sites.