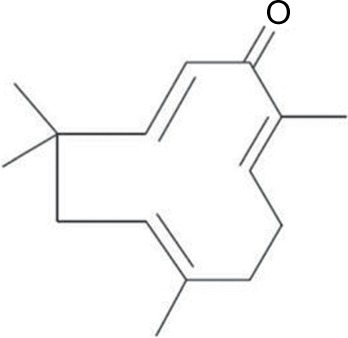
Figures & data
Figure 2 Changes in the body weights of mice in different treatment groups throughout the experimental period of 14 days.
Abbreviations: ANOVA, analysis of variance; CCI, chronic constriction injury.
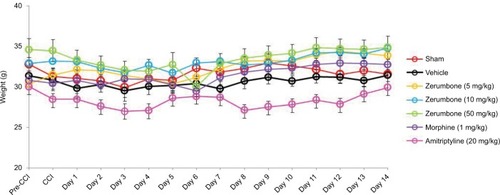
Figure 3 Effect of treatments on the (A) ipsilateral and (B) contralateral paw withdrawal thresholds toward mechanical allodynia as tested with von Frey filament test.
Abbreviation: ANOVA, analysis of variance.
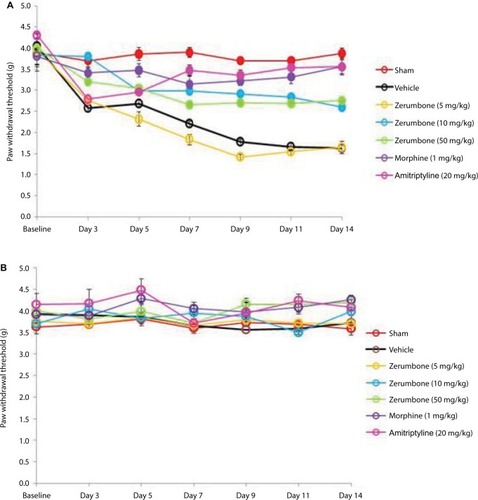
Figure 4 Effect of treatments on cold allodynia withdrawal response as tested with cold plate.
Abbreviation: ANOVA, analysis of variance.
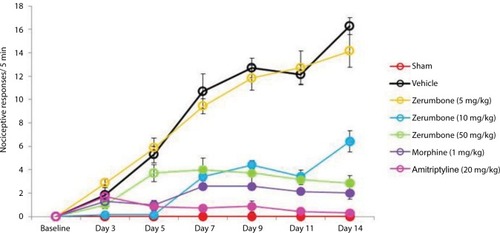
Figure 5 Effect of treatments on the (A) ipsilateral and (B) contralateral paw withdrawal latency toward thermal hyperalgesia as tested with Hargreaves.
Abbreviation: ANOVA, analysis of variance.
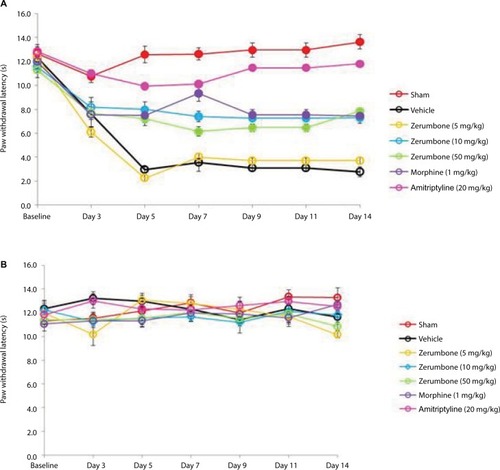
Figure 6 Effect of treatments on the (A) ipsilateral and (B) contralateral paw withdrawal threshold toward mechanical hyperalgesia as tested with Randall–Selitto in CCI and sham mice.
Abbreviations: ANOVA, analysis of variance; CCI, chronic constriction injury.
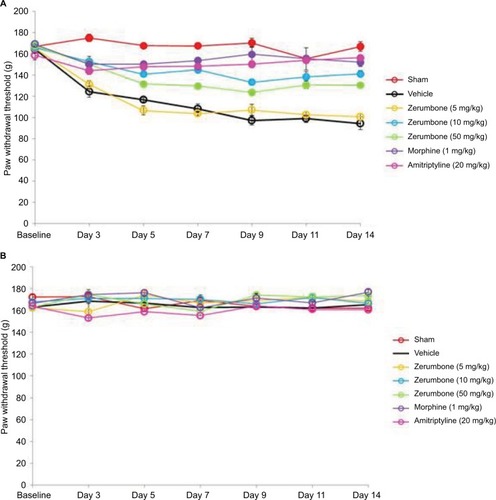
Figure 7 Effect of the treatments on the rotarod performance in CCI and sham mice.
Abbreviation: CCI, chronic constriction injury.
Figure 8 Effect of zerumbone (10 and 50 mg/kg) treatments on the blood plasma levels of (A) IL-1β, (B) TNF-α, (C) IL-10, and (D) IL-6 in CCI and sham mice.
Abbreviations: CCI, chronic constriction injury; IL-1β, interleukin-1beta; TNF-α, tumor necrosis factor-alpha.
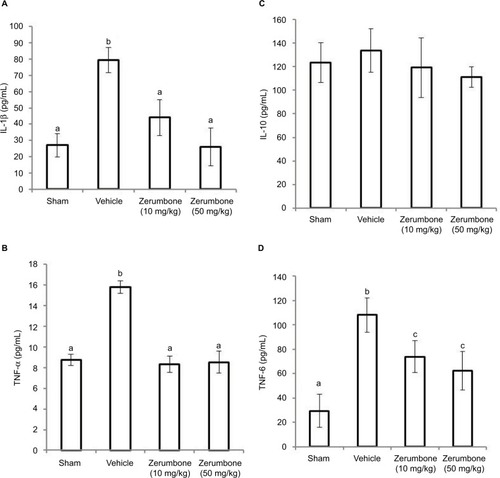
Figure 9 Effect of zerumbone (10 and 50 mg/kg) treatment on the L4–L6 spinal cord levels of (A) IL-1β, (B) TNF-α, (C) IL-10, and (D) IL-6 in CCI and sham mice.
Abbreviations: CCI, chronic constriction injury; IL-1β, interleukin-1beta; TNF-α, tumor necrosis factor-alpha.
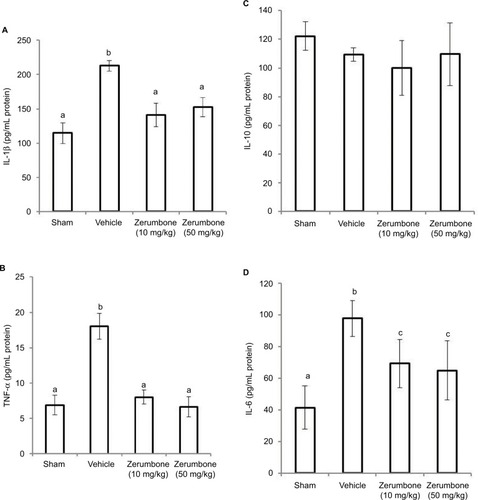
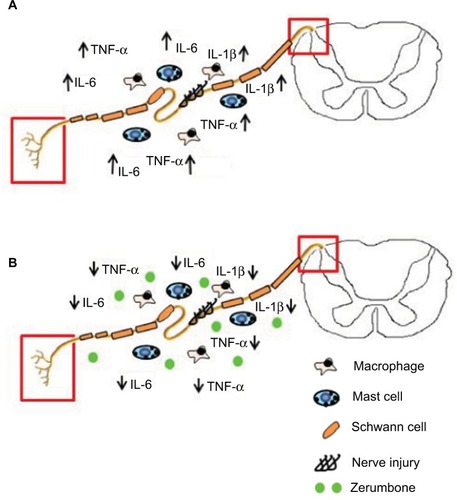
Figure 10 Overview of the antiinflammatory actions of zerumbone on neuropathic pain symptoms allodynia and hyperalgesia.
Abbreviations: IL, interleukin; IL-1β, interleukin-1beta; TNF-α, tumor necrosis factor-alpha.