Figures & data
Figure 1 Effects of cyclopamine on morphine-induced hyperalgesia and tolerance.
Abbreviations: i.p., intraperitoneally; MPE%, percentage of maximal possible effect; Mor, morphine; CLP, cyclopamine; DMSO, dimethyl sulfoxide.
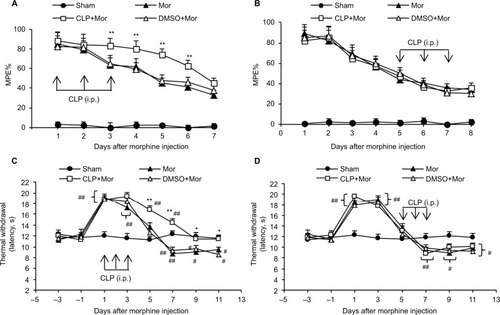
Figure 2 Knockdown of Shh in spinal cord prevents the reduction of chronic morphine treatment-induced MPE%.
Abbreviations: MPE%, percentage of maximal possible effect; siRNA, small interfering RNA; i.t., intrathecally; Mor, morphine; con-siRNA, control-siRNA.
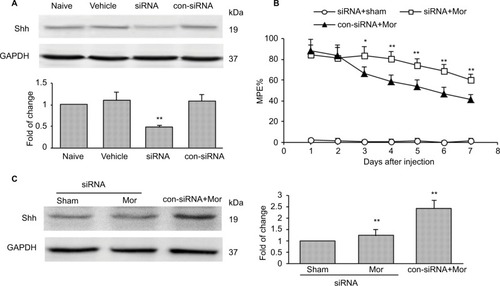
Figure 3 Shh signaling inhibition with cyclopamine suppressed morphine treatment-induced upregulation of c-fos and CGRP in the spinal cord of mice.
Abbreviations: i.p., intraperitoneally; Mor, morphine; CLP, cyclopamine.
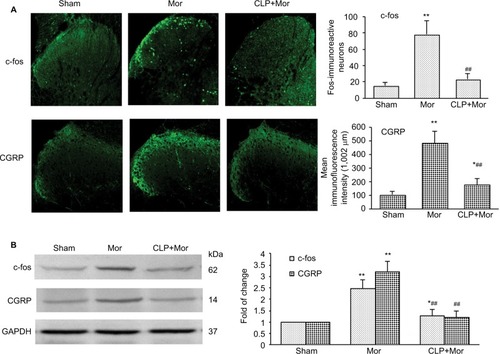
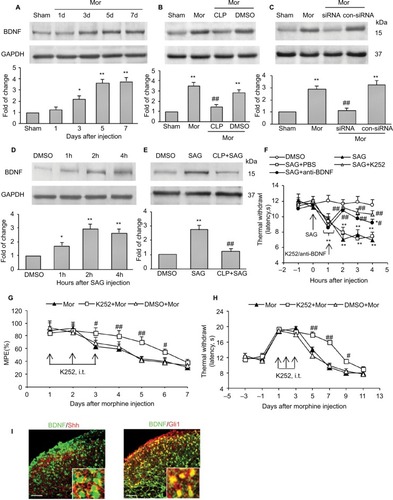
Figure 4 Expression and activation of Shh signaling after chronic morphine treatment.
Abbreviations: SC, spinal cord; DRG, dorsal root ganglion; i.t., intrathecally; Mor, morphine; ELISA, enzyme linked immunosorbent assay.
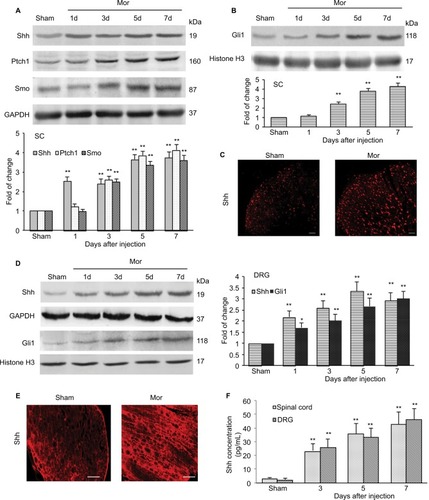
Figure 5 Shh signaling contributed to tolerance and MIH through regulating BDNF expression.
Abbreviations: MIH, morphine-induced hyperalgesia; BDNF, brain-derived neurotrophic factor; siRNA, small interfering RNA; i.p., intraperitoneally; i.t., intrathecally; Mor, morphine; CLP, cyclopamine; DMSO, dimethyl sulfoxide; SAG, smoothened agonist; MPE, maximal possible effect.