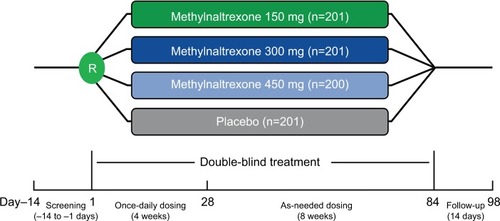
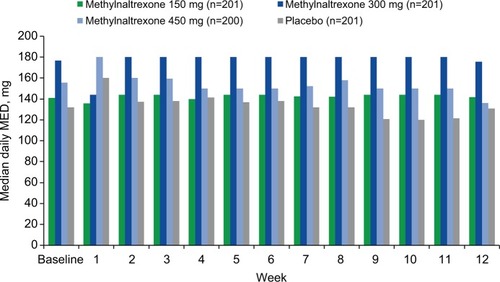
Figures & data
Table 1 Demographic and baseline characteristics
Table 2 TEAEs occurring in ≥2% of patients in any treatment group, n (%)
Table 3 Occurrence of AE of interest by oral methylnaltrexone dose
Table 4 Drug-related treatment-emergent adverse eventsTable Footnotea occurring in ≥1% of patients in any treatment group, n (%)
Table 5 TEAEs in the cardiac disorder SOC, n (%)
Figure 3 Mean OOWS scores over time (A) with cramping item and (B) without cramping item.
Abbreviation: OOWS, Objective Opioid Withdrawal Scale.
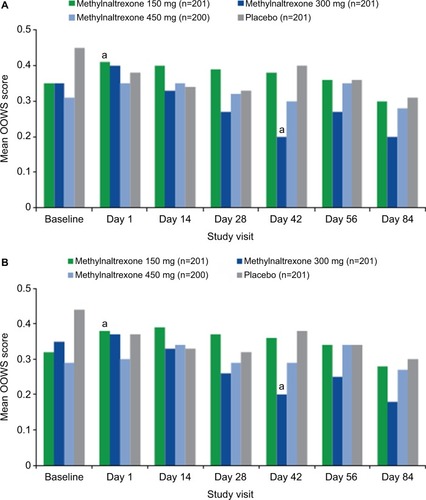
Figure 4 Mean SOWS scores over time (A) with cramping item and (B) without cramping item.
Abbreviation: SOWS, Subjective Opioid Withdrawal Scale.
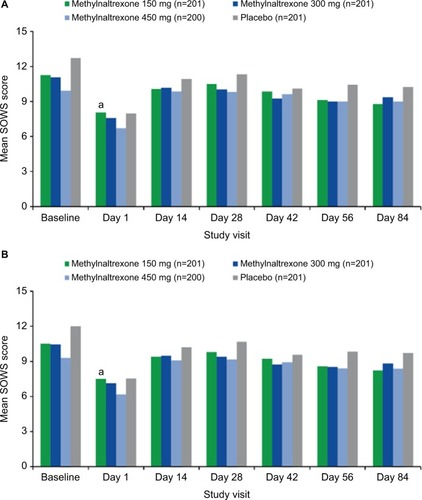
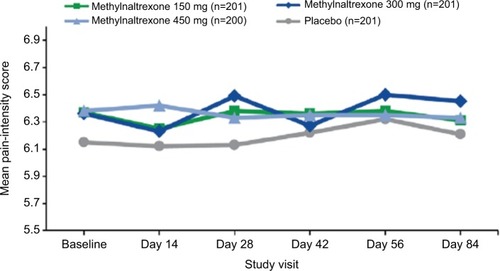
Figure 5 Mean pain-intensity scores over time.