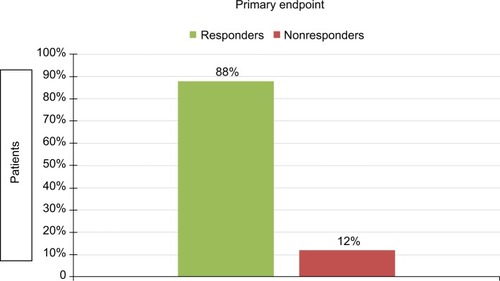
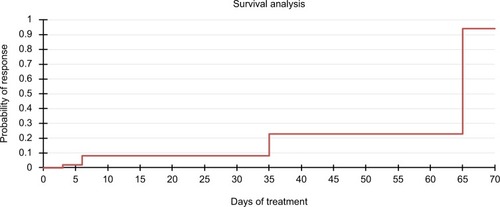
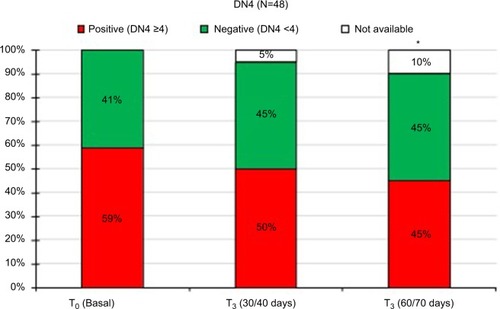
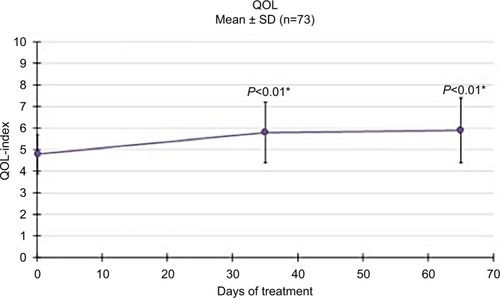
Figures & data
Table 1 Inclusion and exclusion criteria
Table 2 Baseline demographic and clinical data (n=80)
Figure 2 Regression analysis showed that no covariate tested was significant.
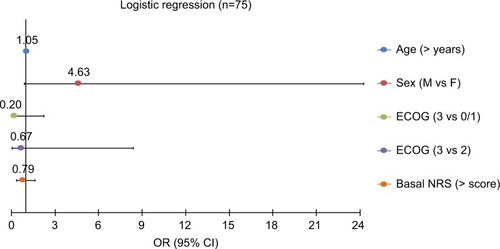
Figure 4 Pain intensity.
Abbreviation: NRS, numeric rating scale.
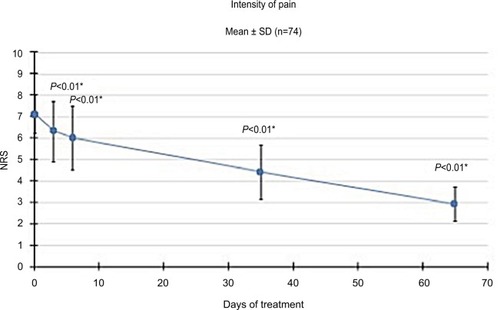
Figure 5 Pain-intensity differences.
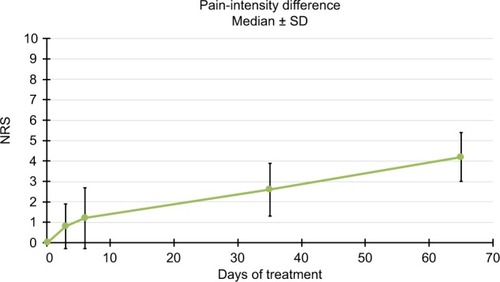
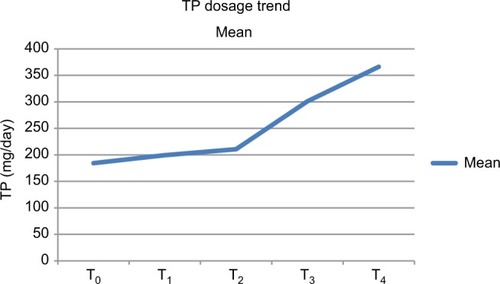
Figure 6 TP-dosage trend.
Abbreviation: TP, tapentadol.