Figures & data
Table 1 Properties of tapentadol
Figure 1 Differential contribution of μ-opioid agonism and noradrenaline reuptake inhibition in acute and chronic neuropathic pain models.Citation30 In acute pain, antagonizing μ-opioid agonism with naloxone moves the dose–response curve further to the right than antagonizing noradrenaline reuptake inhibition with yohimbine, showing that μ-opioid agonism makes a greater contribution to the compound’s analgesic effect. In chronic neuropathic pain, the opposite is true; noradrenaline reuptake inhibition contributes more to analgesia.
Reprinted from European Journal of Pain, vol 14, issue 8. Schröder W, De Vry J, Tzschentke TM, Jahnel U, Christoph T. Differential contribution of opioid and noradrenergic mechanisms to the antinociceptive and antihypersensitive efficacy of tapentadol in rat models of nociceptive and neuropathic pain, 814–821, Copyright (2010), with permission from Elsevier.
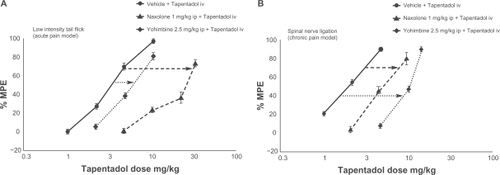
Figure 2 Average pain intensity scores over time.Citation39 Pain relief was consistent during the maintenance period for both active treatment groups. Tapentadol PR significantly reduced mean pain intensity compared with placebo at week 12 and throughout the maintenance period, using the last observation carried forward imputation method for missing values.
Used with permission of Informa Healthcare, from Expert Opinion on Pharmacotherapy, Buynak R, et al, Vol 11, Issue 11, 2010; permission conveyed through Copyright Clearance Center, Inc.
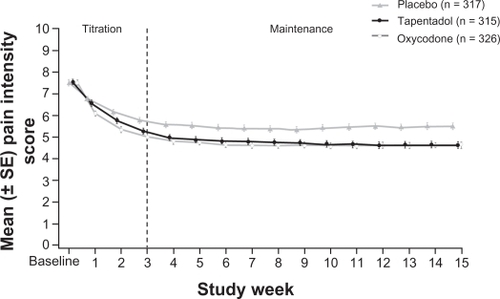
Figure 3 Treatment discontinuations over time.Citation39 The time to discontinuation in the tapentadol PR group was significantly longer than in the oxycodone CR group (P < 0.001), but not significantly different from that in the placebo group (P = 0.309). The primary reason for treatment discontinuation in the active treatment groups was adverse events (16.7% of the tapentadol PR group, compared with 32.3% of the oxycodone CR group).
Used with permission of Informa Healthcare, from Expert Opinion on Pharmacotherapy, Buynak R, et al, Vol 11, Issue 11, 2010; permission conveyed through Copyright Clearance Center, Inc.
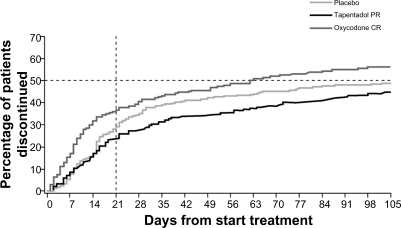
Figure 4 Weekly average pain intensity scores.Citation40 During the double-blind maintenance period, the mean pain intensity increased in the placebo group but remained stable in the tapentadol PR group. Tapentadol PR also showed a favorable tolerability profile, with a maximum of about 20% for individual adverse events.
Used with permission of Informa Healthcare, from Current Medical Research and Opinion, Schwartz S, et al, Vol 27, Issue 1, 2011; permission conveyed through Copyright Clearance Center, Inc.
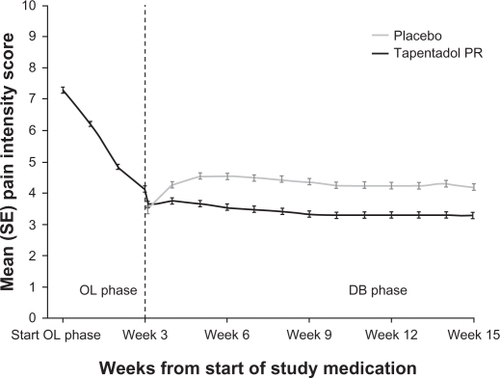
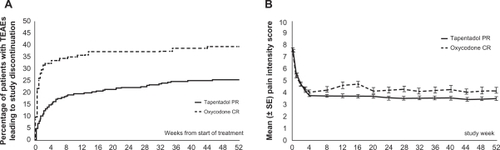
Figure 5 Discontinuations and mean pain intensity scores over time in patients suffering from low back pain and osteoarthritis.Citation41,Citation42 A) The percentage of patients who discontinued treatment because of adverse events was lower for tapentadol than for oxycodone (22.7% vs 36.8%). The overall percentage of patients who discontinued treatment was also lower for tapentadol than for oxycodone (53.8% vs 65%). B) Tapentadol PR provided sustainable relief of moderate to severe chronic knee or hip osteoarthritis or low back pain for up to 1 year. The efficacy of tapentadol PR was comparable to that of oxycodone CR.
Figure 5B from Pain Practice, Vol 10, Wild et al. Copyright © 2010 by John Wiley & Sons, Inc. Reprinted by permission of John Wiley & Sons, Inc; permission conveyed through Copyright Clearance Center, Inc.