Figures & data
Figure 1 Trial design.
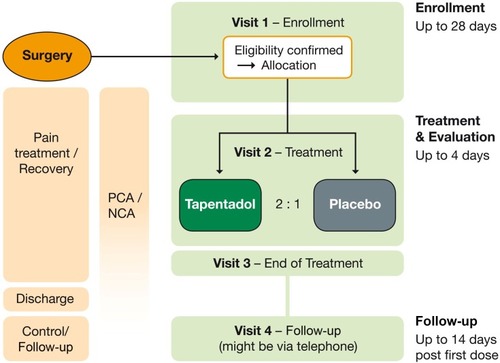
Table 1 Efficacy Outcome Measures
Figure 2 Patient flow chart.
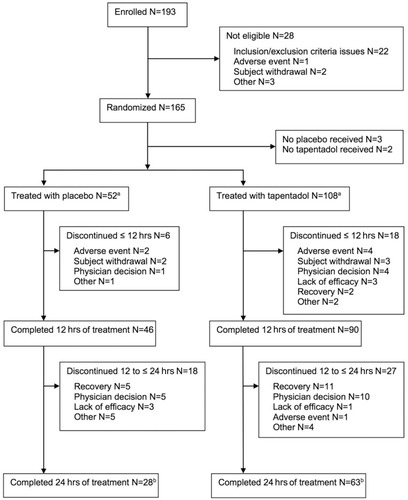
Table 2 Demographic Data And Baseline Characteristics
Figure 3 Least squares mean difference (95% CI) between the trial medications for the amount of supplemental opioid analgesic medication used within the first 24 hrs after intake of first trial medication.
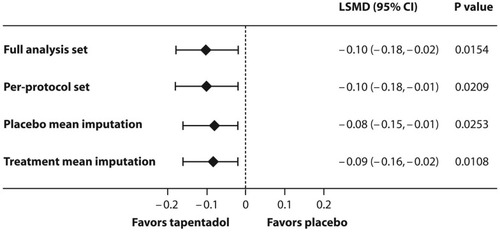
Figure 4 Mean pain intensity (±95% confidence interval) prior to each intake of trial medication (first seven intakes). (A) Face, Legs, Activity, Cry, and Consolability scale; (B) Faces Pain Scale-revised; (C) Visual Analog Scale.
Abbreviations: FLACC, Face, Legs, Activity, Cry, and Consolability; FPS-R, Faces Pain Scale-revised; VAS, Visual Analog Scale.
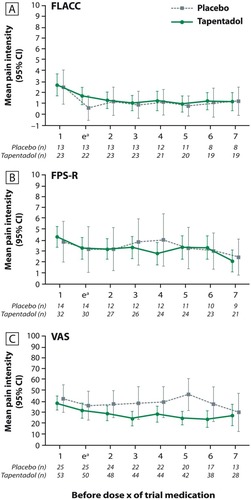
Figure 5 Palatability (taste) and acceptance (swallowing) of the trial medication after first and last doses (full analysis set).
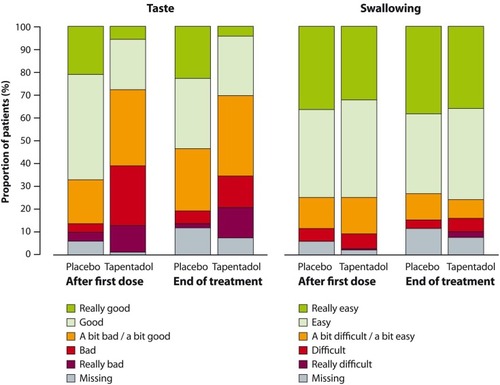
Figure 6 Clinical Global Impression of Change and Patient Global Impression of Change after completion of double-blind treatment (full analysis set).
Abbreviations: CGIC, Clinical Global Impression of Change; PGIC, Patient Global Impression of Change.
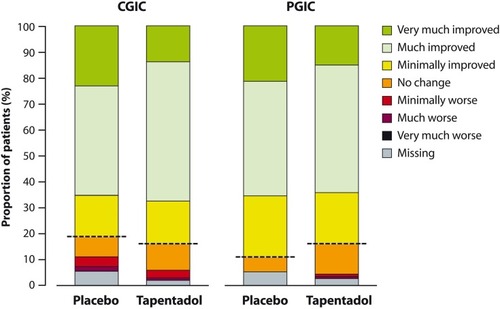
Table 3 Treatment-Emergent Adverse Event Profile Of The Trial Population (Safety Set)
