Figures & data

Table 1 Characteristics of Lidocaine Topical System 1.8% and Lidocaine Patch 5%
Table 2 Demographics and Baseline Characteristics (Study 1)
Table 3 Demographics and Baseline Characteristics (Study 2)
Table 4 Pharmacokinetic Parameter Estimates of Lidocaine in Plasma (per Protocol Population) (Studies 1 and 2)
Figure 1 Mean lidocaine plasma concentrations versus time profiles for IV lidocaine 0.7 mg/kg, semilog scale (N = 56) (Study 1). Time = 0 is pre-dose measurement.
Abbreviation: IV, intravenous.
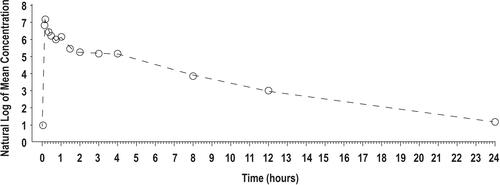
Figure 2 Mean lidocaine plasma concentrations versus time profiles for lidocaine topical system 1.8% (N = 56) and lidocaine patch 5%, semilog scale (N = 54) (Study 1). Time = 0 is pre-dose measurement.
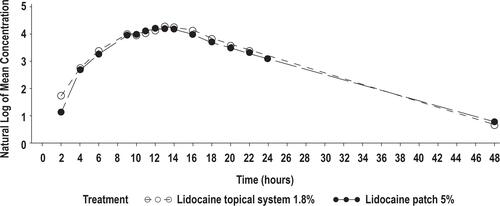
Table 5 Analysis of Bioequivalence (Study 1)
Table 6 Analysis of Bioequivalence (Study 2)
Figure 3 Mean lidocaine plasma concentrations versus time profiles for lidocaine topical system 1.8% and lidocaine patch 5% (Study 2).
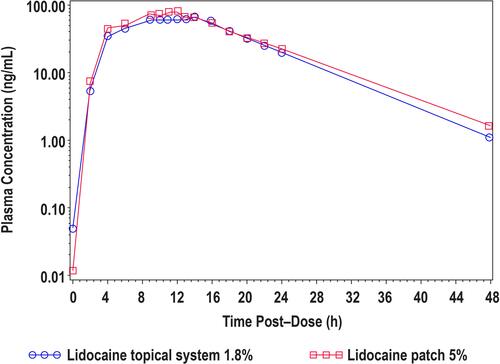
Table 7 Summary of Apparent Dose Absorbed (Studies 1 and 2)
Table 8 Summary of Adhesion (Study 2)
Table 9 Summary of Irritation Analysis (Study 2)
Table 10 Overall Summary of Adverse Events (Study 1)
Table 11 Overall Summary of Adverse Events (Safety Population) (Study 2)
