Figures & data
Figure 1 Interactions at the central neuroimmune interface. Sensory neurons after injury or high-stimulation activation releases mediators from the presynaptic central terminal in the dorsal horn of the spinal cord to engage cognate receptors on immunocompetent cells in the central nervous system (CNS) such as microglia and astrocytes. Colony-stimulating factor 1 (CSF1) binds to CSF1 receptor (CSF1R), neuregulin 1 (NRG1) binds to tyrosine-protein kinase (ErbB2), CX3-C-chemokine ligand 1(CX3CL1) binds to CX3C receptor 1 (CX3CR1), matrix metalloprotease 9 (MMP9) and caspase 6 (CASP6) both facilitate the cleavage of Pro-interleukin-1 beta (IL-1b) into the mature IL-1b, fibroblast growth factor 6 (FGF6) binds to FGF receptors (FGFRs), CXC chemokine ligand 3 (CXCL3) binds to CXC receptor 5 (CXCR5), and damage associated molecular patterns (DAMPs), which include adenosine triphosphate (ATP; which can bind to purinoceptors (P2X, P2Y)), heat shock proteins (HSP) 70, HSP90, fibronectin, high mobility group box 1 (HMGB1) that all bind to toll-like receptors (TLR) 2 and 4. Unique and shared soluble mediators from neurons, immune, and immunocompetent cells of the spinal cord form feedback and feedforward signaling mechanisms which affect the nociceptive excitability and contribute to pain behavior: IL-1b binds to IL-1 receptor (IL-1R), IL-6 binds to IL-6 receptor (IL-6R), prostaglandin E2 (PGE2) binds to PGE receptor (PGER), tumor necrosis factor alpha (TNFa) binds to TNF receptor (TNFR). Activated microglia release cathepsin S (CatS) which further cleaves CX3CL1. Activate astrocytes are identified by the increased expression of glial fibrillary acidic protein (GFAP). Activated astrocytes increase Connexin 43 (Cx43) hemichannels which allows for extracellular release of CXCL1 (binding to CXCR2 on postsynaptic dorsal horn neuron), C-C chemokine ligand 2 (CCL2; binding to CCR2 on neurons), and glutamate (binding to α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartic acid (NMDA) glutamatergic receptors). D-serine, a potent co-agonist of the NMDA receptor is also released from activated astrocytes.
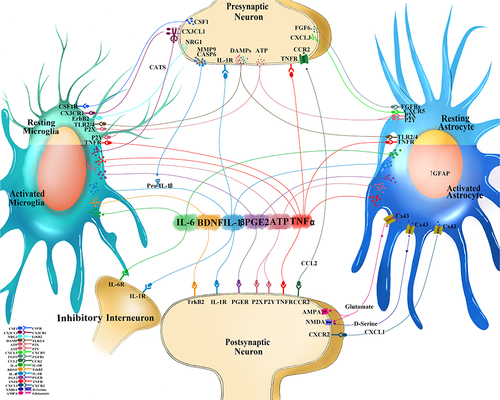
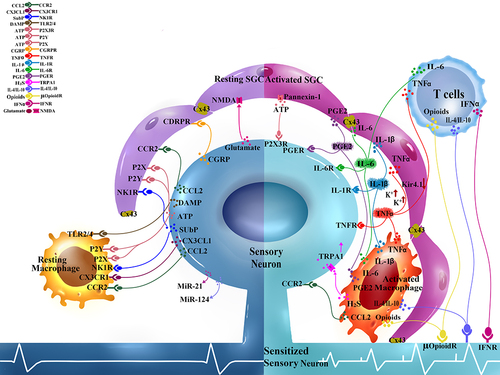
Figure 2 Interactions at the peripheral neuroimmune interface. Sensory neurons cell bodies and satellite glial cells (SGC) form tight neuron-glial units in the dorsal root ganglion (DRG). Soluble mediators are released from the sensory neuron soma and readily bind to receptors on SGC and also serve as chemoattractant for macrophages and T cells: Glutamate activate N-methyl-D-aspartic acid (NMDA) glutamatergic receptors, substance P (SP) binds to neurokinin 1 receptor (NK1R), C-C motif chemokine ligand 2 (CCL2) binds to C-C motif chemokine receptor 2 (CCR2), calcitonin gene-related peptide (CGRP) binds to CGRP receptors (CGRPR), adenosine triphosphate (ATP) binds to various purinoceptors (P2X, P2Y), damage associated molecular patterns (DAMPs) bind to toll-like receptors (TLR2, TLR4). Injured sensory neurons also release microRNAs (MiR-21 and miR-124 which can have pro-inflammatory and anti-inflammatory effects). Activated SGC, T cells, and macrophages in the DRG respond with shared and unique mediators that in turn bind to receptors on the DRG sensory neurons to affect neuronal excitability. Interleukin-1-beta (IL-1b) binds to IL-1 receptor (IL-1R), IL-6 binds to IL-6 receptor (IL-6R), prostaglandin E2 (PGE2) binds to PGE receptor (PGER), tumor necrosis factor alpha (TNFa) binds to TNF receptor (TNFR). Activated SGC increase Connexin-43 (Cx43) gap junctions between adjacent cells to allow for increased electrical coupling. Activated SGC also increase extracellular channels such as Pannexin-1, which allows for ATP release. Inward rectifying potassium channels (Kir4.1) are downregulated in activated SGC which results in increased extracellular potassium gradient and making neurons hyperexcitable. Activated macrophage produce hydrogen sulfide (H2S) that can activate neuronal transient receptor potential cation channel A1 (TRPA1). Infiltrating T cells depending on the subtype can contribute to pro-excitatory neuronal and is a source of interferon gamma (IFNg) which can bind to IFN receptor (IFNR). Subtypes of macrophage and T cells can contribute to anti-inflammation by releasing IL-4, IL-10, and opioids.