Figures & data
Table 1 Patient Demographics
Figure 1 The study schematic. Patients were enrolled and evaluated for baseline pain scores on day one of the studies. Two- and four-week follow-ups were included to evaluate compliance.
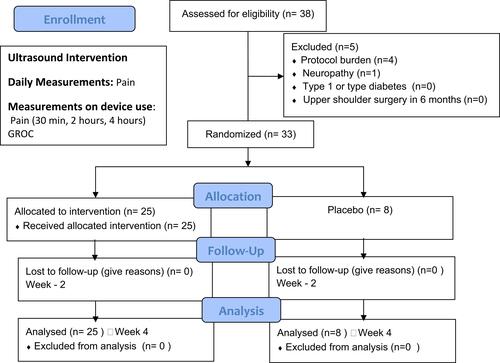
Figure 2 Wearable long duration ultrasound device (SAM®, ZetrOZ Systems LLC, Trumbull, CT) bilateral placement. If patients were experiencing bilateral trigger points, a transducer was placed on each side over the trigger point. If the patient experienced unilateral trigger points, only one transducer was used. The transducer was placed over the most painful trigger point if the patient was experiencing multiple trigger points on one side.
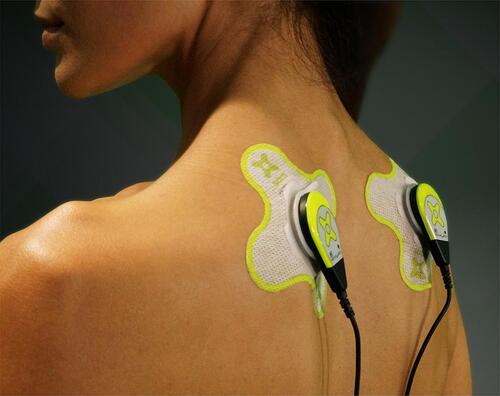
Table 2 Primary Outcomes. The Pain Reported at Baseline and Weekly Averages. Change in Pain from Baseline and Comparison Between Groups Evaluated Using Independent t-Tests
Table 3 The Pain Reported Before, 30 Minutes into the Treatment, 2 Hours into Treatment, and Post-Treatment (4 Hours). Change in Pain from Pre-Treatment and Comparison Between Groups Evaluated Using Independent t-Tests
Table 4 The Pain Reported Before, 30 Minutes into the Treatment, 2 Hours into Treatment and Post-Treatment (4 Hours). Change in Pain from Pre-Treatment and Comparison Between Groups Evaluated Using Independent t-Tests
