Figures & data
Figure 1 Patient disposition.
Note: aOne event each of angina pectoris, hepatitis E, myelitis, and somnolence.
Abbreviations: AE, adverse event; CLCR, creatinine clearance.
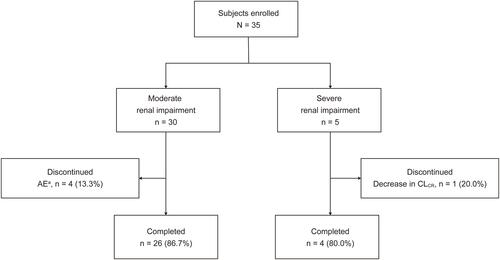
Figure 2 Study design.
Notes: aAfter obtaining informed consent, patients who were treated with prohibited concomitant medications (DPNP and PHN) or prohibited concomitant therapies (PHN) underwent a washout period of ≥7 days.
Abbreviations: BID, twice daily; CLCR, creatinine clearance; DPNP, diabetic peripheral neuropathic pain; PHN, post-herpetic neuralgia; QD, once daily.
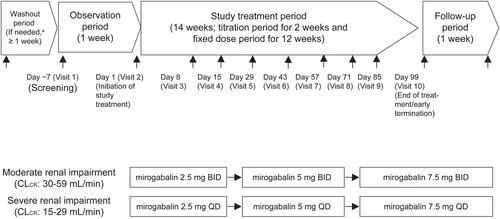
Table 1 Demographic and Other Baseline Characteristics (Safety Analysis Set)
Table 2 Summary of TEAEs and ADRs (Safety Analysis Set)
Table 3 Efficacy Outcomes (Efficacy Analysis Set)
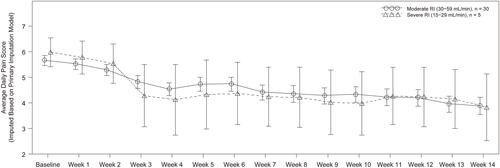
Figure 3 Time course of average daily pain scoresa (efficacy analysis set).
Notes: aData are shown as least squares mean ± SE. The MI method using pattern mixture model with different shifting parameters according to the reason for treatment discontinuation was applied. The MMRM with CLCR group, week and CLCR group-by-week as fixed effects and baseline ADPS as a covariate was performed to estimate the least squares means and the corresponding SE for each week.
Abbreviations: ADPS, average daily pain score; CLCR, creatinine clearance; MI, multiple imputation; MMRM, mixed-effects model with repeated measures; RI, renal impairment; SE, standard error.