Figures & data
Table 1 Baseline Characteristics
Table 2 Underlying Diagnosis/Reason for Pain
Figure 1 Patient flow chart. (A) Randomized treatment phase, (B) tapentadol extension phase, (C) safety observation.
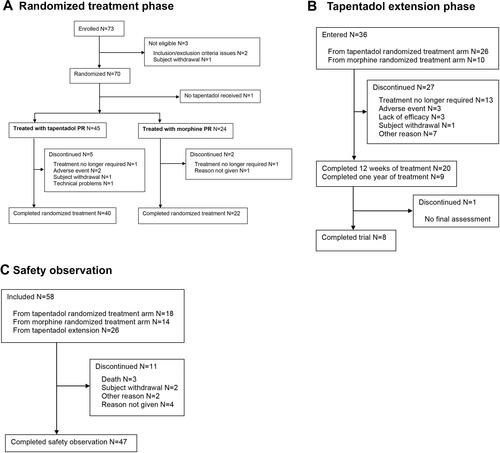
Figure 2 Mean change in pain intensity (SD) from baseline to the last six assessments before final administration of trial medication (randomized treatment phase).
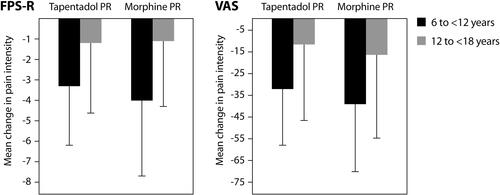
Figure 3 Forest plot of primary endpoint analysis and sensitivity analyses of the difference in treatment response between tapentadol PR and morphine PR (14-day randomized treatment period).
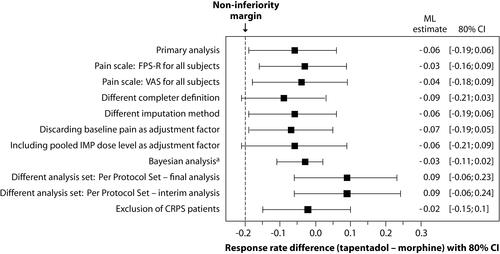
Figure 4 Mean pain intensity under long-term tapentadol PR treatment (“as observed” data).
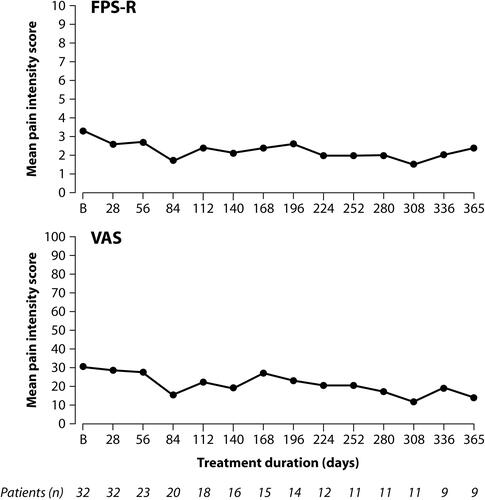
Table 3 Treatment-Emergent Adverse Event Profile During the Randomized Treatment Period (Safety Set)
Table 4 Treatment-Emergent Adverse Event Profile During the Tapentadol PR Extension Phase (Safety Set, n=36)
