Figures & data
Figure 1 Injury sites of each peripheral nerve injury model. The sciatic nerve branches into the tibial nerve, peroneal nerve, and sural nerve. The sural nerve includes only sensory fibers. In model 1, the sciatic nerve was ligated and resected (blue cross). In model 2, the sural nerve was resected (yellow cross). In model 3 (the spared nerve injury [SNI] model), both motor nerves were resected and the sensory nerve was preserved (red cross). In model 4, the animals underwent a sham operation. Only the SNI models showed mechanical hypersensitivity. Three weeks after the surgery, we isolated lumbar dorsal root ganglia (L3, 4, and 5) and compared DNA expression patterns among the groups.
![Figure 1 Injury sites of each peripheral nerve injury model. The sciatic nerve branches into the tibial nerve, peroneal nerve, and sural nerve. The sural nerve includes only sensory fibers. In model 1, the sciatic nerve was ligated and resected (blue cross). In model 2, the sural nerve was resected (yellow cross). In model 3 (the spared nerve injury [SNI] model), both motor nerves were resected and the sensory nerve was preserved (red cross). In model 4, the animals underwent a sham operation. Only the SNI models showed mechanical hypersensitivity. Three weeks after the surgery, we isolated lumbar dorsal root ganglia (L3, 4, and 5) and compared DNA expression patterns among the groups.](/cms/asset/5bbd5ef3-b27c-462e-834b-b99c3f5f4f58/djpr_a_12170567_f0001_c.jpg)
Figure 2 Venn diagram of nerve injury models. The right oval area subtracted from the center circle was extracted as spared nerve injury-specific genes ((A), upregulated; (B), downregulated). SNI, spared nerve injury. (C) Of the 50 SNI-specific genes showing an increase, 2 were validated using real-time PCR. Cdk1 and Avpr1a were significantly increased compared with the sham model.
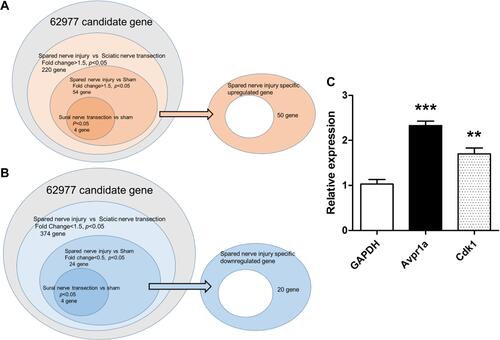
Table 1 SNI-Specific Upregulated Genes (n = 50) Extracted by Analysis of DNA Microarray Results
Table 2 SNI-Specific Downregulated Genes (n = 20) Extracted by Analysis of DNA Microarray Results
Figure 3 V1a expression in the dorsal root ganglia (L3, 4, and 5) 3 weeks and 6 weeks after SNI surgery. The SNI model showed a significant 2.23±0.8-fold increase in expression at 3 weeks. The SNI model also showed a significant 1.53±0.34-fold increase at 6 weeks.
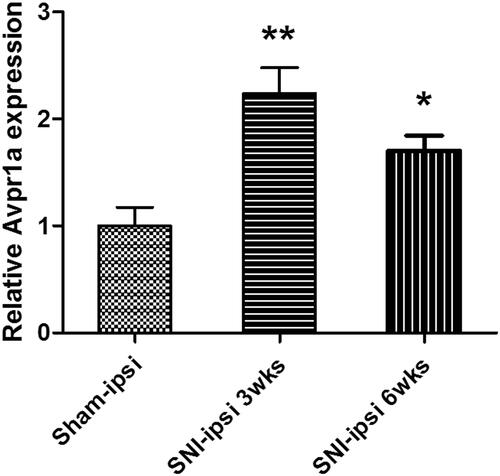
Figure 4 Dorsal skin sensitivity changes. Changes in the mechanical flexor reflex withdrawal response to stimulation of the dorsal surface of the hindpaw (sural nerve territory) after spared nerve injury (SNI) (n = 8) and sham (n = 8) procedures. The withdrawal threshold of the dorsal skin had a higher threshold in the control period and a smaller reduction after the SNI. This change was similar in vasopressin receptor 1A knockout SNI mice.
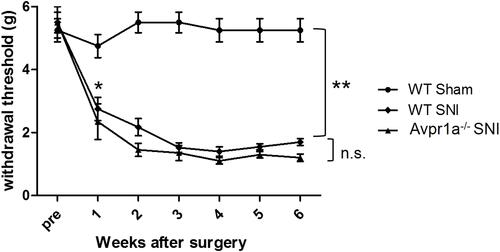
Figure 5 Changes in mechanical stimulation hypersensitivity with vasopressin receptor 1A (V1a) agonist. The groups intraperitoneally administered 0.5 mg/kg and 1.0 mg/kg V1a selective agonist showed a significant increase in the threshold of the mechanical stimulation response 30 min after administration compared with the phosphate-buffered saline group for both males (A) and females (B). The change in the threshold 30 and 60 min after administration tended to be volume-dependent. Similar results were seen in spared nerve injury models 6 weeks after injury in both males (C) and females (D). There were no significant changes in the response to mechanical stimulation in male (E) or female (F) V1a knockout mice at any dose.
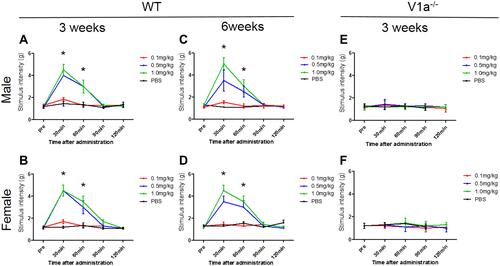
Figure 6 Changes in mechanical stimulation hypersensitivity induced by V1a antagonist. Wild-type (WT) sham (A) and spared nerve injury (SNI) mouse (B) groups 3 weeks after surgery. Neither the WT SNI nor sham mice of either sex showed any apparent mechanical hypersensitivity 30, 60, 90, or 120 min after administration.
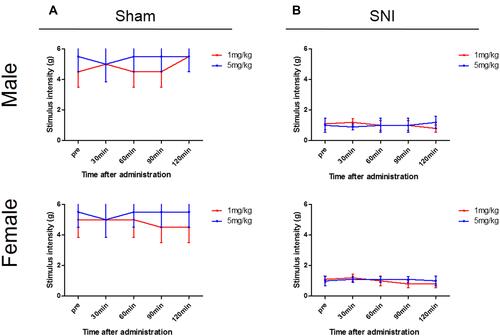
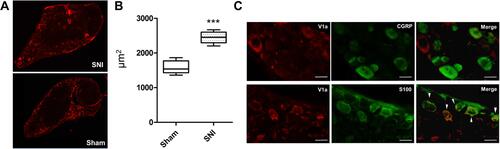
Figure 7 Immunohistochemistry of the lumbar dorsal root ganglia with vasopressin receptor 1A (V1a) antibody. (A) V1a was mainly expressed in the rim of DRG cells in both SNI and sham-operated animals. (B) A significant difference was found in the cross-sectional area of V1a expression between SNI mice (2445±46.2 μm2) and the sham-operated model (1582 ± 50.7 μm2, p<0.001). (C) Double-immunostaining assay showed that V1a colocalized with S-100 (arrowheads), but not with CGRP.