Figures & data
Table 1 Effect of ASP0819 on Membrane Potential in Human and Rat KCa3.1 Channel-Expressing CHO Cells
Figure 2 Effect of ASP0819 on muscular withdrawal responses in RIM rats. Myalgia was induced by treatment with SC reserpine for 3 days. Vehicle or ASP0819 (0.1, 0.3, and 1 mg·kg-1) was orally administered 8 days later, and the muscle pressure threshold (MPT) was measured 4 h after administration. Data are expressed as the mean ± SEM in each group (n=6). #P<0.05, statistically significant compared to the normal group (Student’s t-test). *P<0.05, statistically significant compared to the vehicle group (Dunnett’s multiple comparisons test).
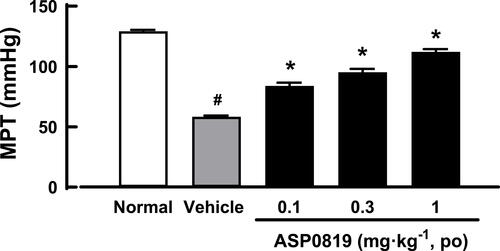
Figure 3 Effect of ASP0819 on muscular withdrawal responses in VIM rats (A) 4 h after a single dose and (B) after repeated daily doses across 14 days. (A) The effect of ASP0819 was evaluated in a rat vagotomy model after a single administration. One week after the sham or vagotomy operation, vehicle or ASP0819 (0.1, 0.3, and 1 mg·kg-1) was orally administered, and the muscle pressure threshold (MPT) was measured 4 h after administration. (B) The effect of ASP0819 was evaluated in a rat vagotomy model after repeated administration. Vehicle or ASP0819 (0.1, 0.3, and 1 mg·kg-1) was orally administered once a day for 14 days from one week after the sham or vagotomy operation, and the MPT was measured at 2, 4, 24, and 48 h after the final administration. Data are expressed as the mean ± SEM in each group (n=6). #P<0.05, statistically significant compared to the normal group (Student’s t-test). *P<0.05, statistically significant compared to the control group (Dunnett’s multiple comparisons test).
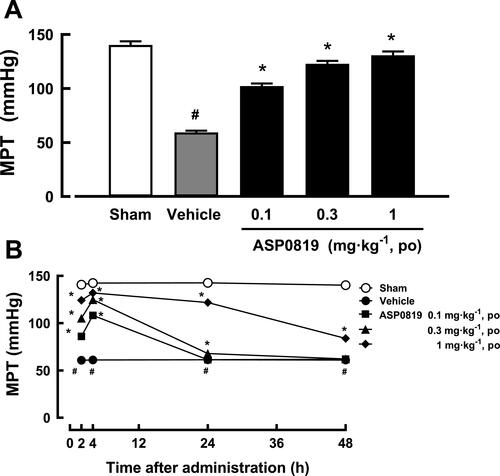
Figure 4 In vivo electrophysiology in a rat RIM model. Spontaneous and mechanically-evoked nerve firing in single primary sensory neurons of the dorsal root were recorded in urethane-anesthetized RIM rats (A). Percent changes in spontaneous and mechanically-evoked nerve firing rate in Aδ-fiber are shown in (B and C), respectively. Data are expressed as the mean ± SEM (n=8–10) in each group. #P<0.05, statistically significant compared to the vehicle-treated group at same time point (Student’s t-test). *P<0.05, statistically significant compared to the vehicle-treated group at same time point (Dunnett’s multiple comparisons test).
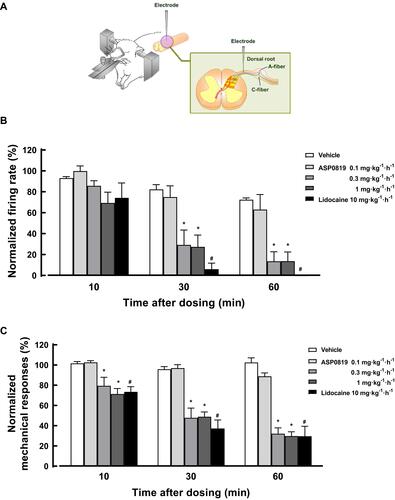
Figure 5 Effect of ASP0819 on withdrawal threshold in a rat AIA model. Arthritis was induced by administering complete Freund’s adjuvant into the footpad of the right hind paw of rats. The withdrawal threshold (mmHg) was measured using the Randall-Selitto method 4 h after administration of vehicle, ASP0819 (0.3, 1, and 3 mg·kg-1), or the positive control drug (diclofenac: 1 mg·kg-1). Data are expressed as the mean ± SEM in each group (n = 8). #P<0.05, statistically significant compared to the vehicle-treated group (Student’s t-test). *P<0.05, statistically significant compared to the vehicle-treated group (Dunnett’s multiple comparisons test).
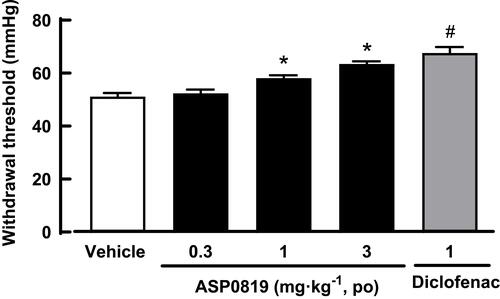
Figure 6 Effect of oral administration of ASP0819 on weight distribution in MIA model rats. Rats were given a single intraarticular injection of 1 mg sodium monoiodoacetate or saline (normal) through the infrapatellar ligament of the right knee. From 23 to 27 days after the injection, vehicle or ASP0819 was orally administered as a single daily dose. At 23 (single administration; (A) and 27 (repeated administration for 5 days; (B) days, hind paw weight distribution was measured 2 h after administration using an incapacitance tester. The data represent the mean ± SEM weight distribution (n = 8). #P<0.05, statistically significant compared to the normal group (Student’s t-test). *P<0.05, statistically significant compared to the vehicle-treated group (Dunnett’s multiple comparisons test).
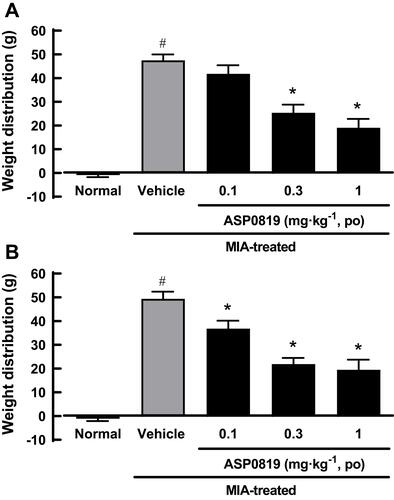
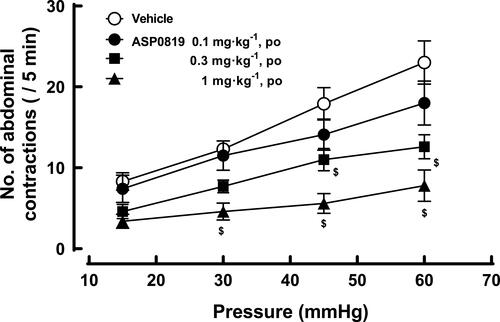
Figure 7 Effect of ASP0819 on visceromotor response induced by colorectal distension in rats. One hour after vehicle or ASP0819 (0.1–1 mg·kg-1) was orally administered, the number of abdominal contractions induced by balloon distension from 15 to 60 mmHg at 15-mmHg steps was counted. Each distension period was 5 min and was performed at 5-minute intervals. Data are expressed as the mean ± SEM in each group (n=10). $P<0.05, statistically significant compared to the control group (Dunnett’s multiple comparisons tests with Bonferroni correction).