Figures & data
Table 1 Characteristics of Studied Lidocaine Topical Products
Table 2 Subject Demographics and Baseline Characteristics
Table 3 Mean Adhesion Scores Using FDA Scales for Lidocaine Topical System 1.8%, Lidocaine Patch 5%, and Lidocaine Medicated Plaster (Studies 1, 2, and 3)
Figure 1 Adhesion assessments at the end of the dosing period (12 hours). The proportion of subjects with at least 90% adhesion (using the FDA adhesion rating scale) at the end of the 12-hour application period and the proportion of subjects who had experienced 0% adhesion (complete detachment) at any time point throughout the study in (A) Study 1 (n=54); (B) Study 2 (n=44); and (C) Study 3 (n=24).
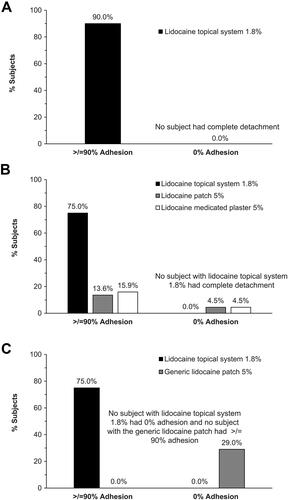
Table 4 Mean Percent Adhesion Over Time for Lidocaine Topical System 1.8% and Lidocaine Patch/Plaster 5% Comparators (Studies 2 and 3)
Figure 2 Mean percent adhesion over time. Mean percent adhesion scores were evaluated every 3 hours over the 12-hour application period. (A) In Study 2, there was a significant difference favoring the lidocaine topical system 1.8% over the lidocaine patch 5% and lidocaine medicated plaster 5% at baseline and at each time point after application (P<0.0001). (B) In Study 3, there was a significant difference favoring the lidocaine topical system 1.8% over the generic lidocaine patch 5% at baseline and at each time point after application (P<0.0001 for all comparisons).
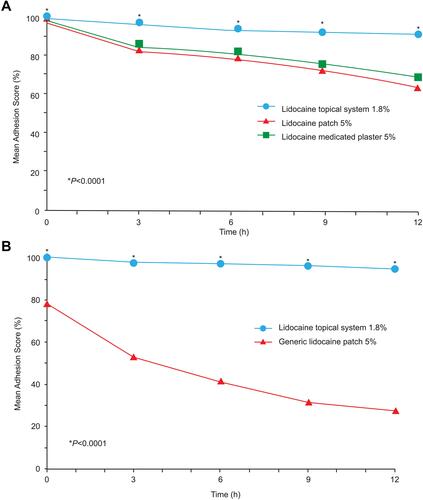
Figure 3 Representative adhesion performance with the FDA score (denoted in red) in Studies 2 and 3. Subjects were treated with the lidocaine topical system 1.8% (top rows in panels A and B), lidocaine patch 5% (middle row in A), lidocaine medicated plaster 5% (bottom row in A), or generic lidocaine patch 5% (bottom row in B). Photographs were taken immediately following product application (0 hours) and at the end of the study after 12 hours (±15 minutes) of wear.
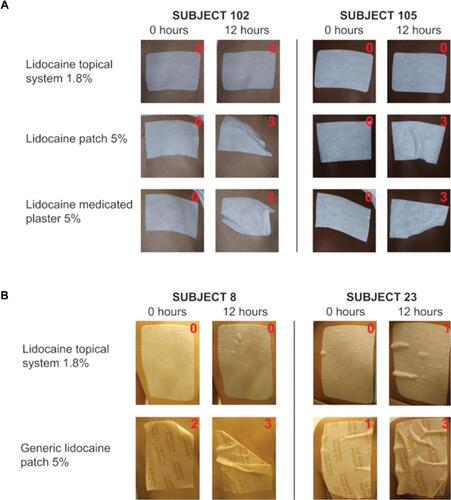
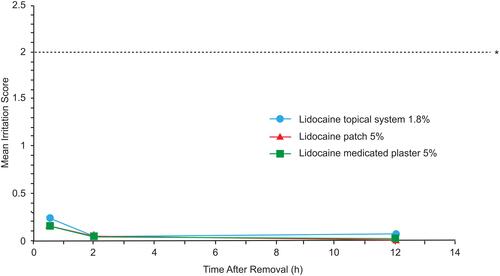
Figure 4 Mean irritation scores after product removal in Study 2. Irritation at the application site was assessed at 0.5 hours (+10 minutes), 2 hours (±15 minutes), and 12 hours (±30 minutes) after product removal. Irritation was graded using an 8-point scale of dermal response . Overall mean scores across all time points were not significantly different between the lidocaine topical system 1.8% and lidocaine patch 5% or lidocaine medicated plaster 5% (P=0.1656 for both comparisons). *Horizontal line represents a score of 2, defined as definite erythema, minimal edema, or minimal papular response and is considered clinically meaningful irritation.