Figures & data
Figure 1 Mechanism of Action of Buprenorphine at Opioid and NOP Receptors. At µ-opioid receptors, buprenorphine is a partial agonist with very high binding affinity, which results in potent analgesia, contributes to a ceiling effect on respiratory depression and euphoria, and reduces other adverse events commonly observed with opioid use owing to unique phosphorylation and signaling activity. Buprenorphine has antagonistic activity with high binding affinity at κ- and δ-opioid receptors, which may limit constipation, respiratory depression, dysphoria, and substance abuse. The agonistic activity and low binding affinity at the NOP receptor contribute to spinal analgesia and may limit the substance abuse potential and tolerance commonly observed with full µ-opioid receptor agonists.
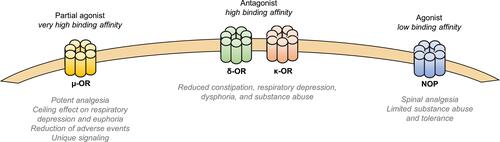
Table 1 Buprenorphine Products
Figure 2 Pain Relief Induced by Intramuscular Morphine or Sublingual Buprenorphine Following Surgery. Pain scores were determined using a VAS after the administration of 0.4 mg SL buprenorphine or an injection of 10 mg/mL morphine. *p<0.05 for comparisons between groups at that time.
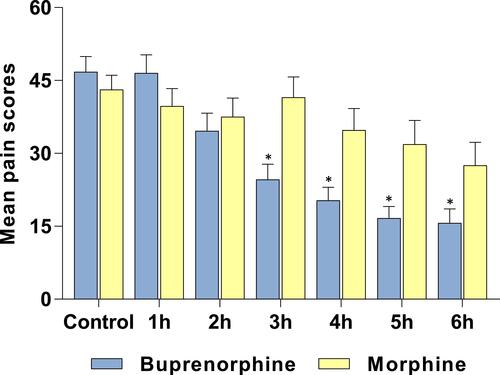
Figure 3 Efficacy of Transdermal Buprenorphine Compared With Conventional Opioids in Patients With Chronic Cancer Pain. Average pain intensity was measured on a numeric rating scale. Data are mean (SD). Data from Corli et al (2016).Citation45
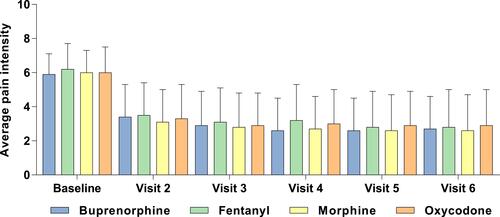
Figure 4 Efficacy of Buprenorphine Buccal Film in Patients With Chronic Low Back Pain. Mean NRS scores during the titration and long-term treatment phases with buprenorphine buccal film in (A) de novo patients and (B) rollover patients.
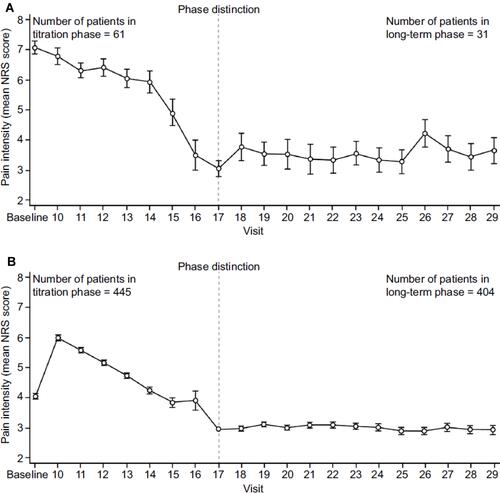
Figure 5 Efficacy of the Transdermal and Buccal Film Formulations of Buprenorphine. Responder analysis of similar opioid-experienced chronic pain clinical trials. Comparisons are of efficacy data for transdermal buprenorphine (20 μg/h) and buprenorphine buccal film (150–900 μg/12h) with response defined as (A) ≥30% or (B) ≥50% reduction in pain intensity.
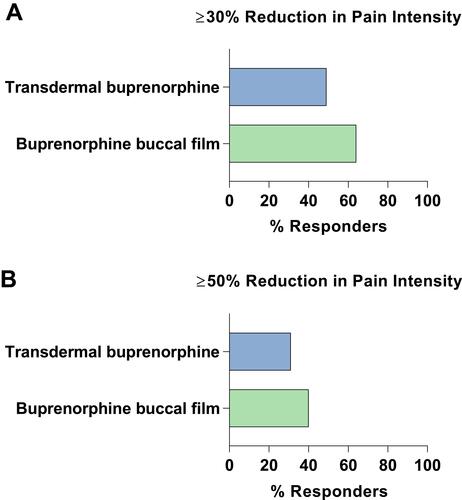
Figure 6 Effect of Buprenorphine Buccal Film and Oxycodone Hydrochloride on Minute Ventilation. Effect of each drug treatment on respiratory drive: mean minute ventilation over time. In the partial completer population (n=16), mean minute ventilation for BBF was not significantly different from placebo at any time point. *p<0.05, **p<0.01, ***p<0.001.
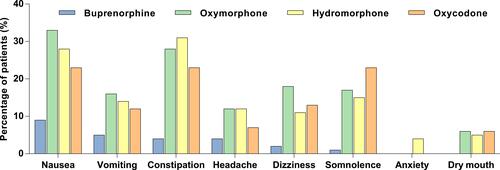
Figure 7 Adverse Events Reported in Clinical Trials of Buprenorphine Buccal Film Compared With Conventional Opioids for Chronic Pain. The percentage of patients who reported adverse events in clinical trials for buprenorphine buccal filmCitation21 compared with those reported for extended-release formulations of oxymorphone,Citation87 hydromorphone,Citation88 and oxycodone.Citation69