Figures & data
Figure 1 Flow charts of in vivo experiments: Experiments 1, 2, and 3.
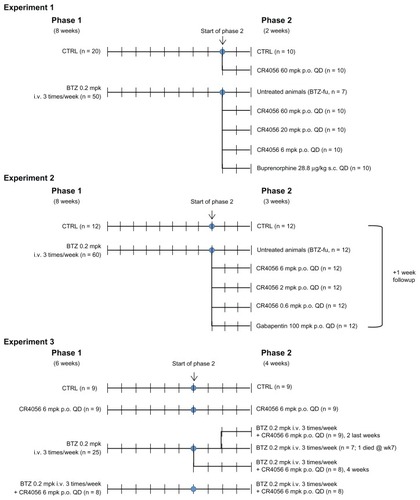
Figure 2 Body weight changes along the study period: Experiment (Exp) 1, phase (A) 1 and (B) 2; in Exp 1, bortezomib (BTZ)-treated animals do not show any significant difference in weight gain with respect to the control (CTRL) at the end of the 8-week treatment. Exp 2, phase (C) 1 and (D) 2; Exp 3, phase (E) 1 and (F) 2; in Exps 2 and 3, BTZ significantly affected body weight growth (C and E) (P < 0.01); CR4056 when coadministered with BTZ neither worsened nor improved the toxicity induced by BTZ (E). No significant changes were observed in each group between the values measured during phase 2 (B, D, and F).
Abbreviations: BTZ-fu, bortezomib-fake-manipulated but untreated; Bupre, buprenorphine; CR4056 0.6, CR4056 at 0.6 mg/kg/day; CR4056 2, CR4056 at 2 mg/kg/day; CR4056 20, CR4056 at 20 mg/kg/day; CR4056 6, CR4056 at 6 mg/kg/day; CR4056 60, CR4056 at 60 mg/kg/day; Gaba, gabapentin; ws, weeks.
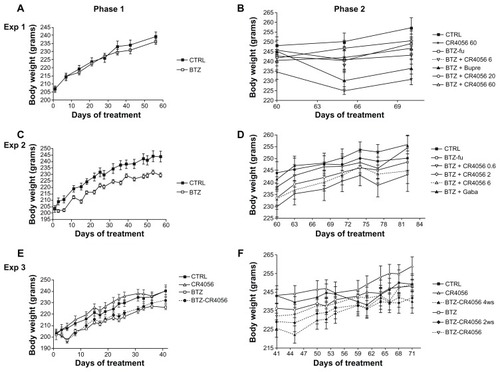
Figure 3 Results of nerve conduction velocity (NCV) study performed on the tail of control (CTRL) and bortezomib (BTZ)-treated rats: Experiment (Exp) 1, phase (A) 1 and (B) 2; Exp 2, phase (C) 1 and (D) 2; Exp 3, phase (E) 1 and (F) 2. BTZ induced a significant reduction in NCV in all BTZ-treated rats after 8 weeks of treatment and at the end of Exps 1, 2, and 3.a The analgesic compounds tested did not significantly counteract the effect of BTZ on NCV.
Abbreviations: BTZ-fu, bortezomib-fake-manipulated but untreated; Bupre, buprenorphine; CR4056 0.6, CR4056 at 0.6 mg/kg/day; CR4056 2, CR4056 at 2 mg/kg/day; CR4056 20, CR4056 at 20 mg/kg/day; CR4056 6, CR4056 at 6 mg/kg/day; CR4056 60, CR4056 at 60 mg/kg/day; Gaba, gabapentin; ws, weeks.
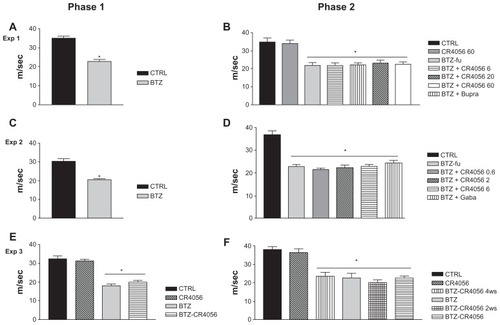
Figure 4 Plantar test phase 1 (A) and 2 (B) in Experiment 1: bortezomib (BTZ) and CR4056 (CR) did not induce any significant modification in thermal threshold with respect to the control (CTRL) rats at any experimental time point evaluated in this study.
Abbreviations: Bupre, buprenorphine; CR 20, CR4056 at 20 mg/kg/day; CR 6, CR4056 at 6 mg/kg/day; CR 60, CR4056 at 60 mg/kg/day; sec, second.
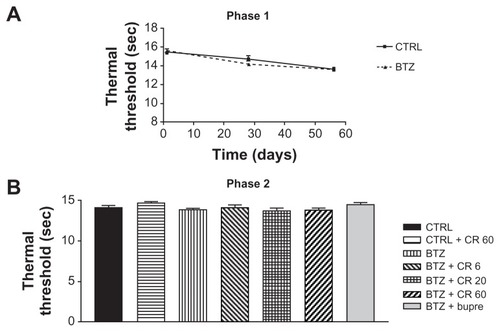
Table 1 Dynamic test data (mechanical nociceptive threshold in grams) at baseline (ie, before starting bortezomib [BTZ] treatment) and at the end of phase 1 (8–6 weeks)Table Footnotea
Figure 5 Dynamic test in phase 2 of (A) Experiment (Exp) 1a; (B) Exp 2b; and (C) Exp 3c. The persistent and significant antiallodynic effect of oral CR4056 6 mg/kg was evident in all studies, in comparison with buprenorphine (Bupre), gabapentin (Gaba), and the other doses of CR4056. Before CR4056 administration, in Exps 1, 2, and 3, bortezomib (BTZ)-treated rats showed a significant difference compared with control (CTRL) (P < 0.05).
Abbreviations: admin, administration; BTZ-fu, bortezomib-fake-manipulated but untreated; CR4056 0.6, CR4056 at 0.6 mg/kg/day; CR4056 2, CR4056 at 2 mg/kg/day; CR4056 20, CR4056 at 20 mg/kg/day; CR4056 6, CR4056 at 6 mg/kg/day; CR4056 60, CR4056 at 60 mg/kg/day; Gaba, gabapentin; IV, intravenously; OS, orally; tiw, three times a week; ws, weeks.
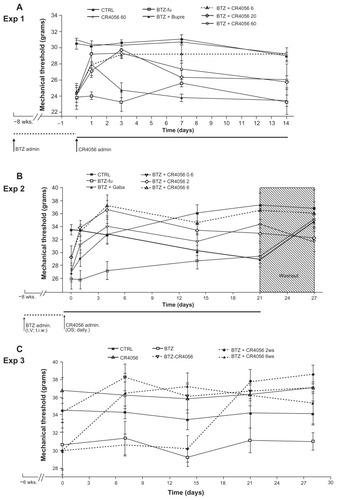
Figure 6 Cytotoxicity effect of bortezomib (BTZ) and CR4056 in (A) A549, (B) H460, (C) RPMI 8226, and (D) H929 cell lines.
Abbreviation: DMSO, dimethyl sulfoxide.
Figure S2. (A) Porsolt forced swimming test: CR4056 (3, 10, and 30 mg/kg) was administered orally (per os) to male CD1 mice 1 hour before the test and both the immobility time and the latency to immobility were evaluated. (B) Tail suspension test: CR4056 was administered per os to male CD1 mice 30 minutes before the test; two independent experiments were performed to cover a broad range of doses (from 0.1 to 30 mg/kg).
Abbreviations: ns, not significant; sec, second.
Notes: *P < 0.05; ** P < 0.01.
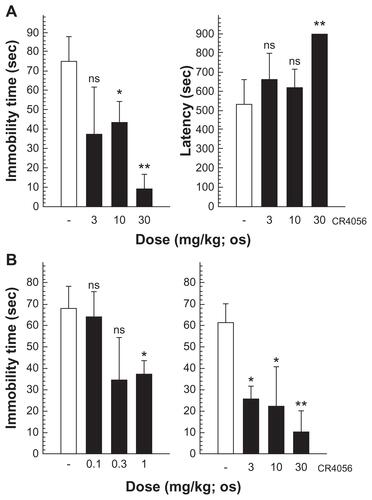
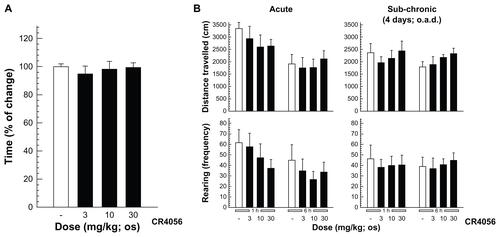
Figure S3. (A) Rota-rod assay: rats were allowed a 30-minute acclimatization period in the testing room and then placed on a 9 cm diameter rod, which increased the speed from 0 to 20 rpm over a 60-second period. The time required for the rat to fall from the rod was recorded, with a maximum score of 60 seconds.a (B) Open field test: CR4056 effects in the open field assay data (evaluated 1 and 6 hours after oral administration) are expressed as the distance traveled and as the rearing activity following an acute (left) and a subchronic treatment period (right).b
Notes: aMean plus or minus standard error of the mean (n = 5 rats/group); bmean plus or minus standard error of the mean (n = 6 rats/group).
Abbreviations: h, hour; os, orally; o.a.d., once a day.