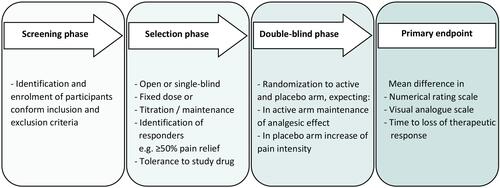
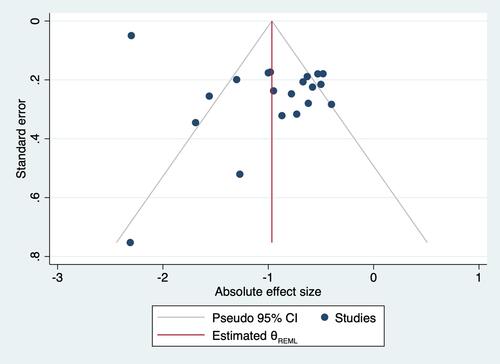
Figures & data
Figure 2 PRISMA flow diagram.
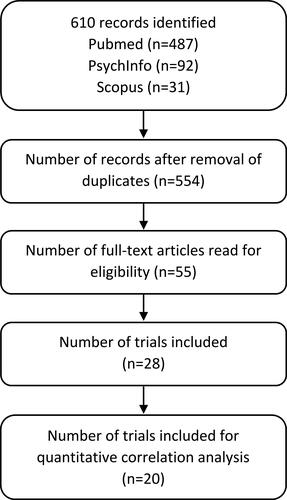
Table 1 Characteristics of EERW Pain Trials with NRS or VAS as Primary Endpoint
Table 2 Characteristics of EERW Pain Trials with Time to LTR & Treatment Failure as Primary Endpoint
Figure 3 Risk of bias summary.
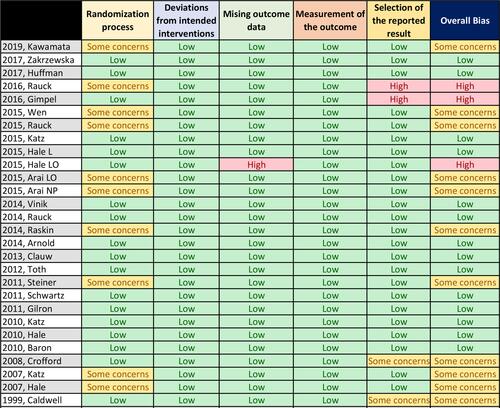
Table 3 Effect Sizes and Treatment-Emergent Adverse Events in the Double-Blind Phase
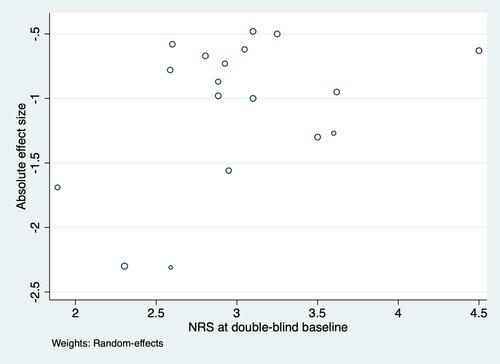
Figure 5 Relation between double-blind baseline NRS and absolute effect size.