Figures & data
Table 1 Primer Sequences used for qRT-PCR.
Figure 1 Effect of PRFE treatment on COX mRNA and prostaglandin D and E synthase expression in HEK and HDF in culture. (A and B) We evaluated the effect of PRFE treatment on levels of mRNA expression after 30 minutes of treatment with PRFE using standard conditions. Cells were harvested 4 hours following initiation of PRFE treatment and the RT-PCR products were analyzed by electrophoresis through 2% agarose gel. (C) Quantitative RT-PCR was used to determine the effect of PRFE on message levels for the various genes involved in prostaglandin synthesis (**P < 0.05 for control versus PRFE treatment) in HDF.
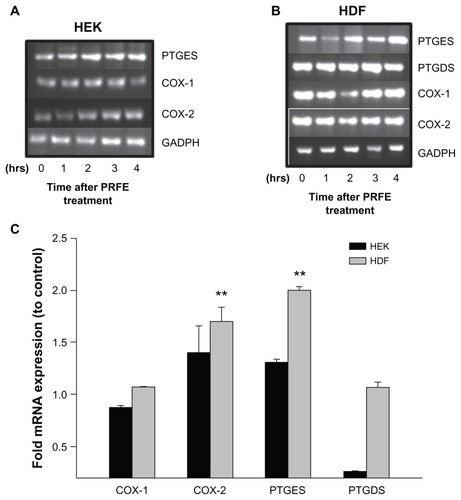
Figure 2 Effect of PRFE fields on COX enzyme activity in HEK and HDF. Total COX enzyme activity was determined after PRFE field treatment. (A) COX was evaluated after PRFE treatment at the indicated times. Data shown are from at least two independent experiments performed in triplicate. (B) Effect at 4 hours following PRFE treatment (n = 6, ***P < 0.01 for HDF).
Abbreviations: CTL, control; COX, cyclooxygenase; HDF, human dermal fibroblasts; HEK, human epidermal keratinocytes; PRFE, pulsed radiofrequency energy.
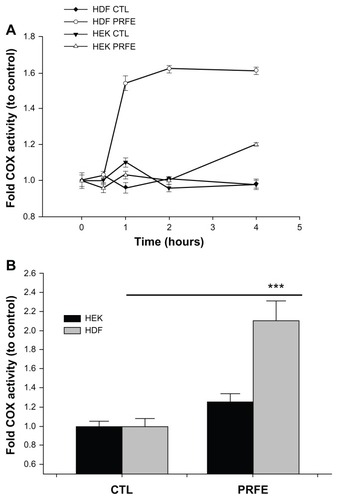
Figure 3 PRFE increases endogenous opioid expression in HEK and HDF at the mRNA and peptide levels. (A) Endogenous opioid expression was determined in HEK and HDF. Cells were treated with PRFE and total RNA was isolated after 2 hours (n = 12, P < 0.01 for all opioids, control versus PRFE-treated). (B and C) Determination of PDYN and PENK opioid levels at 2 hours following PRFE treatment using enzyme-linked immunosorbent assay (error bars show standard error of the mean, two separate experiments in triplicate, P < 0.05).
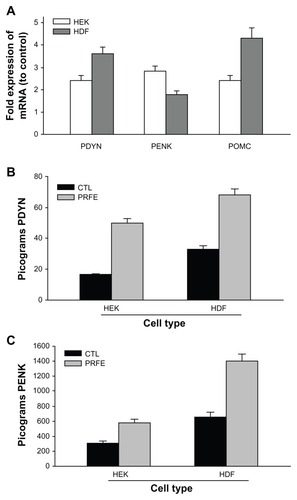
Figure 4 Regulation of cytokines involved in the peripheral pain response.
Abbreviations: HDF, human dermal fibroblasts; HEK, human epidermal keratinocytes; IL, interleukin; NGF-α, nerve growth factor alpha; TNF-α, tumor necrosis factor-alpha; RT-PCR, reverse transcription polymerase chain reaction.
Figure 5 Increased expression of endogenous opioids is regulated by an ET-1/ETB receptor pathway after treatment with PRFE. (A) mRNA expression levels after treatment with PRFE using standard conditions. Cells were harvested after PRFE treatment at the indicated times and RT-PCR products were analyzed by electrophoresis through 2% agarose gel. (B) Quantitative RT-PCR was used to determine the effect of PRFE on message levels of the endogenous opioids, ET-1 and ETB.
Abbreviations: ET, endothelin; GADPH, glyceraldehyde 3-phosphate dehydrogenase; HDF, human dermal fibroblasts; HEK, human epidermal keratinocytes; PRFE, pulsed radiofrequency energy; POMC, pro-opiomelanocortin; PENK, proenkephalin; PDYN, prodynorphin; RT-PCR, reverse transcription polymerase chain reaction.
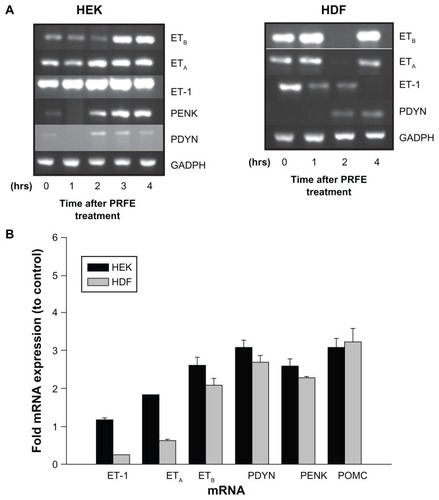
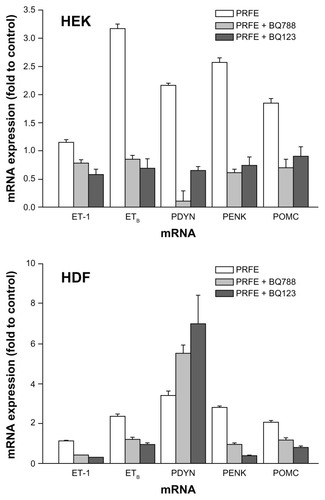
Figure 6 Antagonists of ETA and ETB receptors inhibit opioid expression after PRFE treatment of HEK and HDF.
Abbreviations: ET, endothelin; HDF, human dermal fibroblasts; HEK, human epidermal keratinocytes; PRFE, pulsed radiofrequency energy; POMC, proopiomelanocortin; PENK, proenkephalin; PDYN, prodynorphin; RT-PCR, reverse transcription polymerase chain reaction.