Figures & data
Figure 1 Flow diagram of the randomized clinical trial.
Notes: EDC, refers to the randomization system in the electronic data capture platform; (i. pain score decrease of ≥ 2 and no increase in OMED; ii. pain score no increase and decrease of ≥ 25% in OMED).
Abbreviations: BPI, brief pain inventory; OMED, daily oral morphine equivalents; EORTC QLQ-C30, the European organization for research and treatment of cancer quality of life of cancer patients questionnaire; HAM-D, Hamilton rating scale for depression; HAM-A, Hamilton rating scale for anxiety; WBC, white blood cell count; ANC, absolute neutrophil count; RBC, red blood cell count; PLT, platelet count; CR, complete response (pain score of 0 and no increase in OMED); PR, partial response.
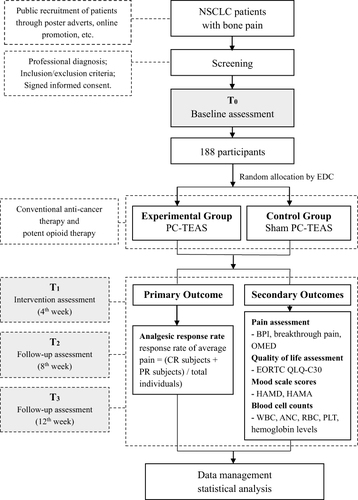
Figure 2 Schematic diagram of transcutaneous electrical acupoint stimulation (TEAS) operation.
Notes: (A) and (B) Patients with pain in sites above the navel, can be treated with the option of bilateral Jiaji acupoint group (cervical / thoracic spine segments) and bilateral upper extremity distal acupoint group (LI4, Hegu - TE5, Waiguan); (C and D) Patients with pain below the navel can be treated with the option of bilateral Jiaji acupoint group (lumbar segments) and bilateral lower extremity distal acupoint group (ST36, Zusanli - SP6, Sanyinjiao).
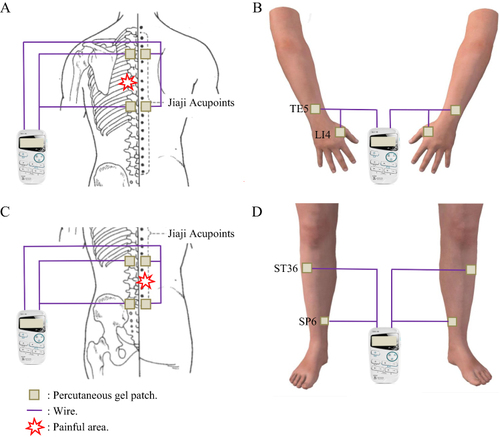
Table 1 SPIRIT - Phases of Trial and Data Collection
