Figures & data
Table 1 Demographics and baseline characteristics, short-term study (safety population)
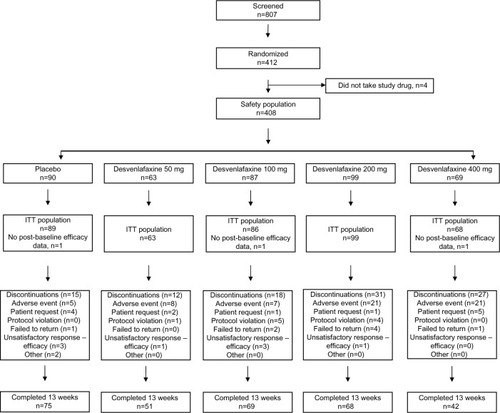
Figure 2 Adjusted mean change from baseline on NRS pain severity over time (ITT; MMRM).
Abbreviations: ITT, intent-to-treat; MMRM, mixed-effects model for repeated measures; NRS, numeric rating scale.
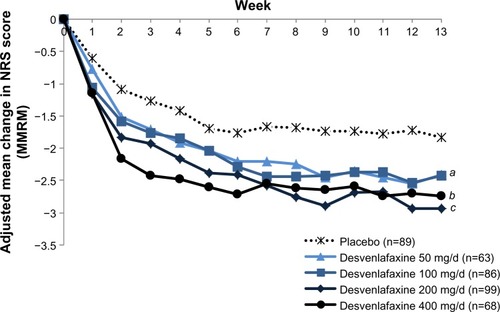
Figure 3 Response rate on pain severity score on the NRS at week 13 (ITT).
Abbreviations: ITT, intent-to-treat; NRS, numeric rating scale.
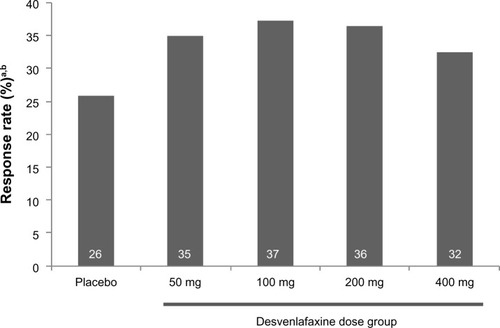
Figure 4 Proportions of patients achieving minimum thresholds of reduction in NRS score at week 13 (ITT).
Table 2 Secondary efficacy endpoints (MMRM), short-term study (intent-to-treat population)
Table 3 Treatment-emergent adverse events in ≥5% and twice that of placebo for patients in any treatment group, short-term study (safety population)
Table 4 Selected vital signs and laboratory values at week 13 (LOCF), short-term study (safety population)