Figures & data
Figure 1 Overview of milnacipran studies.
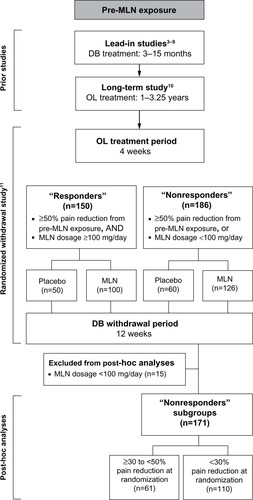
Figure 2 Distribution of pain response at randomization in patients who received milnacipran ≥100 mg/day during the prior long-term, open-label study.
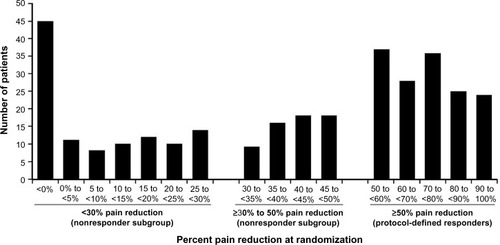
Table 1 Mean scores prior to milnacipran exposure and at study randomizationTable Footnotea
Figure 3 Mean changes from pre-milnacipran exposure in VAS pain scores.
Abbreviations: MLN, milnacipran; VAS, visual analog scale.
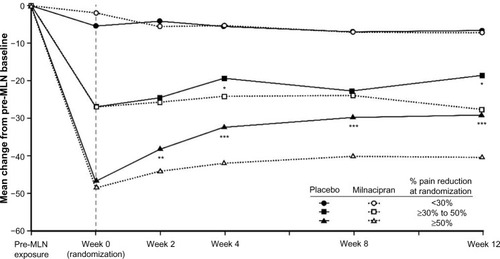
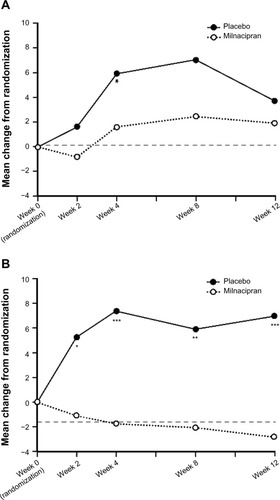
Table 2 Mean changes from randomization to end of the 12-week withdrawal period