Figures & data
Figure 1 Disposition of patients in the study.
Abbreviations: HC-ER, hydrocodone bitartrate extended release; C/T, conversion/titration; AE, adverse event.
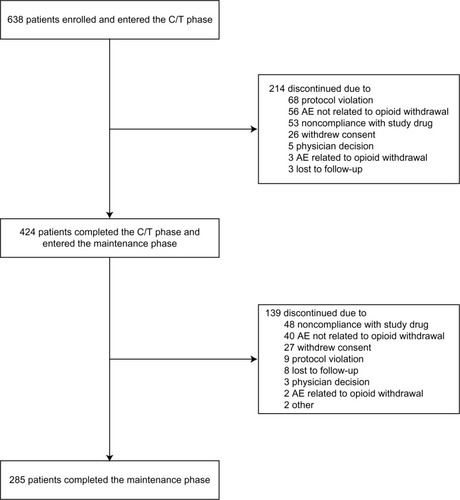
Table 1 Demographic and clinical characteristics at screening for patients who entered the C/T and maintenance phases
Figure 2 HC-ER dose over time during the maintenance phase.
Abbreviation: HC-ER, hydrocodone bitartrate extended release.
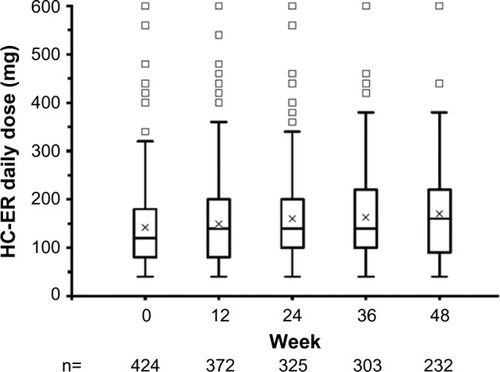
Table 2 Number (%) of patients with treatment-emergent adverse events for patients who entered the C/T and maintenance phases
Figure 3 Mean average pain score from screening to the end of the study for patients who entered the maintenance phase.
Abbreviation: C/T, conversion/titration.
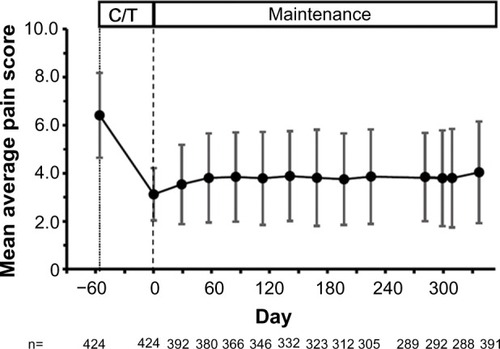
Table 3 HADS and ODI scores of patients who entered the maintenance phase
