Figures & data
Table 1 MSN conversion guide and comparison with previously published equianalgesic data tables
Figure 1 Study flow diagram.
Abbreviation: MSN, extended-release morphine sulfate surrounding sequestered naltrexone hydrochloride.
Figure 2 Frequency (%) of patients achieving stable MSN dose by opioid type.
Abbreviations: ER, extended-release; Excl, excluded; IR, immediate-release; MSN, extended-release morphine sulfate surrounding sequestered naltrexone hydrochloride; Trans, transdermal.
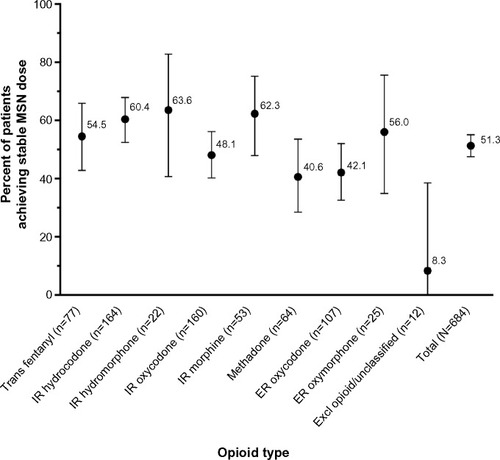
Figure 3 Number of days to reach stable MSN dose by prior opioid.
Abbreviations: ER, extended-release; Excl, excluded; IR, immediate-release; MSN, extended-release morphine sulfate surrounding sequestered naltrexone hydrochloride; Trans, transdermal.
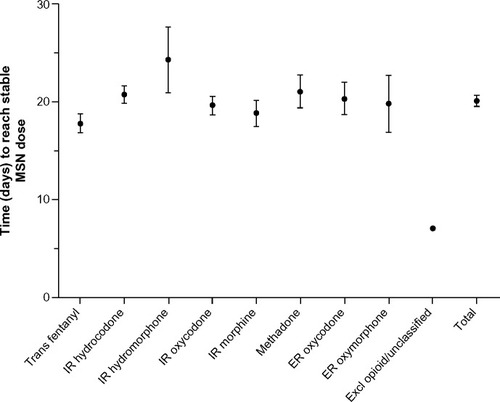
Figure 4 Number of steps to reach stable MSN dose by prior opioid.
Abbreviations: ER, extended-release; Excl, excluded; IR, immediate-release; MSN, extended-release morphine sulfate surrounding sequestered naltrexone hydrochloride; Trans, transdermal.
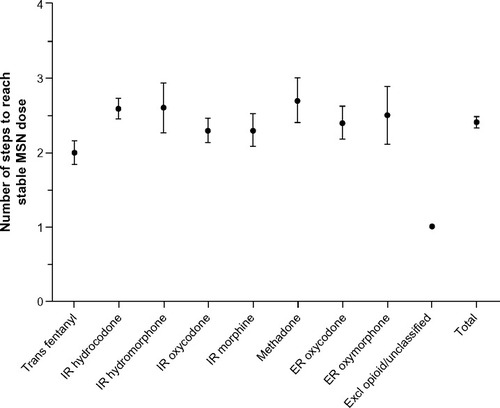
Figure 5 Mean average pain score and mean MSN daily dose in (A) patients achieving a stable MSN dose, and in (B) patients who did not achieve a stable MSN dose.
Abbreviations: MSN, extended-release morphine sulfate surrounding sequestered naltrexone hydrochloride; NA, not available.
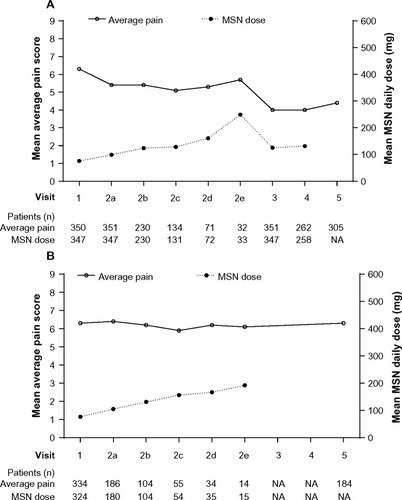
Table 2 Brief Pain Inventory versus activity for patients at baseline (Visit 1) and patients who achieved a stable dose of MSN (Visit 3)
Table 3 Number (%) of patients with treatment-emergent adverse events occurring in >5% of patients (safety population) from any prior opioid type
Table 4 Investigator assessment of MSN conversion guide utility at Visit 3
Table S1 Conversion factors for converting the daily dose of prior oral opioids to the daily oral dose of EMBEDA (mg/day prior opioid × factor = mg/day EMBEDA)
Table S2 Converting from transdermal fentanyl to EMBEDA
