Figures & data
Figure 1 HER2 signaling pathway (Adapted from “HER2 Signaling Pathway”, by BioRender.com (2021). Retrieved from https://app.biorender.com/biorender-templates).
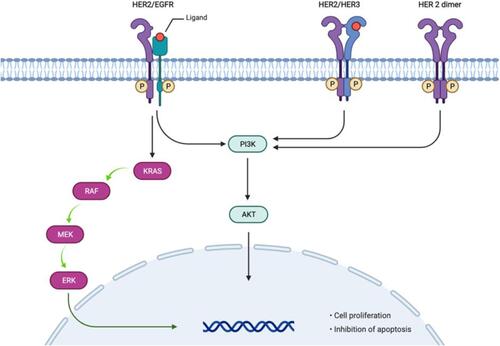
Figure 2 Structure of Trastuzumab Deruxtecan.
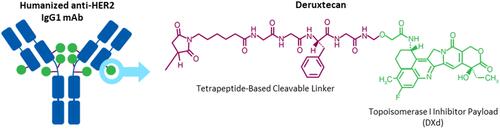
Figure 3 Trastuzumab Deruxtecan mechanism of action (Adapted from “Antibody–Drug Conjugate Drug Release”, by BioRender.com (2021). Retrieved from https://app.biorender.com/biorender-templates).
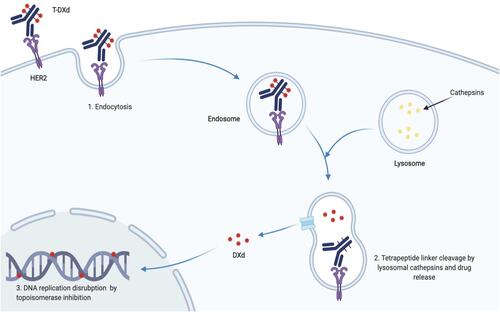
Table 1 Clinical Trials of Trastuzumab Deruxtecan in Non-Small Cell Lung Cancer (NSCLC)
Table 2 Comparison of DESTINY-Lung01 Trial Results Between HER-Mutant and HER2-Overexpressing Cohorts
Table 3 ILD Incidence Rate and Grading Among DESTINY Trials
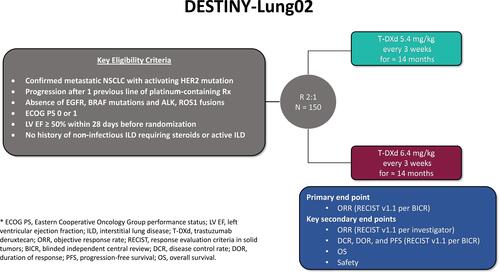
Figure 4 Schema for DESTINY-Lung02. Additional details can be found at: https://clinicaltrials.gov/ct2/show/NCT04644237.