Figures & data
Table 1 Safety features related to falling in ReWalk™, Indego™, and Ekso™ exoskeletons
Table 2 Marketing status of some commercially available devices
Table 3 Inclusion and exclusion criteria in clinical trials
Table 4 Published adverse events involving exoskeletons
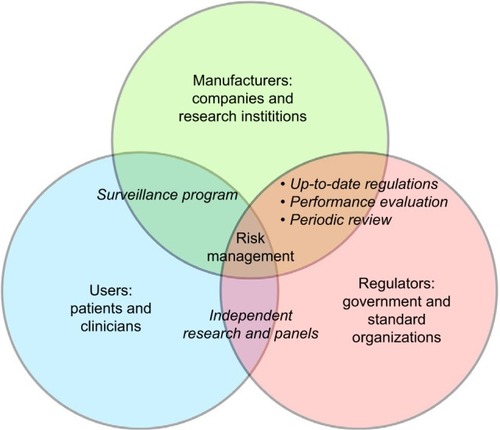
Table 5 Identified risks to health and mitigation measures
Figure 2 Procedures for obtaining medical device approval in the USA, EU, and Japan. The USA requires applications to be approved by a federal agency, namely the FDA, whereas the EU distributes the responsibility to many independent notified bodies. Japan’s government reviews reliability of the manufacturers both on site and via documents, while the USA and the EU leave that responsibility to manufacturers themselves. The bottom panel about Japan was adapted from the diagram on Page 11 in the materials of the 2011 AHC Workshop on Medical Devices. Tamura A. Understanding Japanese medical device requirements. 2011. Available from: https://www.pmda.go.jp/files/000164006.pdf.Citation73
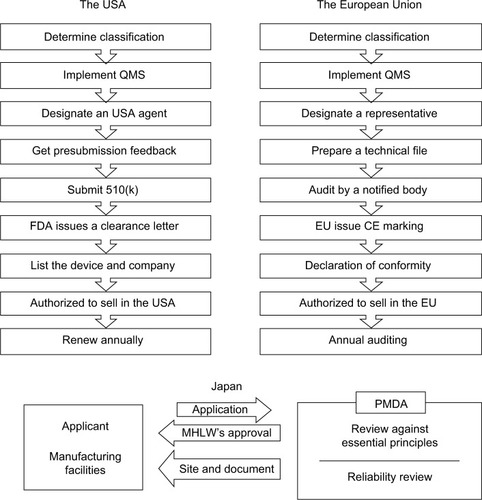
Table 6 Standards recommended by the FDA