Figures & data
Figure 2 Summary of research informing the design and validation of the autoinjector.
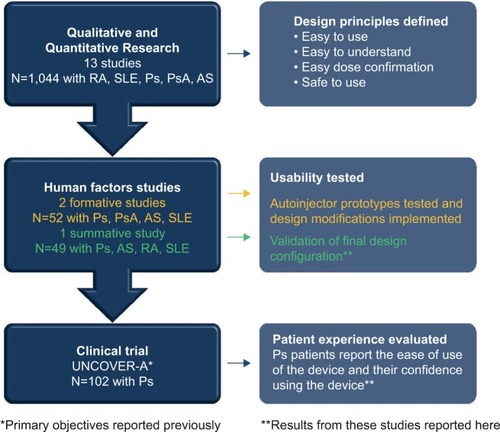
Table 1 Summary of autoinjector features
Table 2 Tasks performed for injection
Table 3 SQAAQ questionnaire
Table 4 Usability study: summary of injection failures in patients and caregivers by training arm
Figure 3 Patient responses to the SQAAQ weeks 0–4 and 8 (observed case data).
Abbreviation: SQAAQ, subcutaneous administration assessment questionnaire.
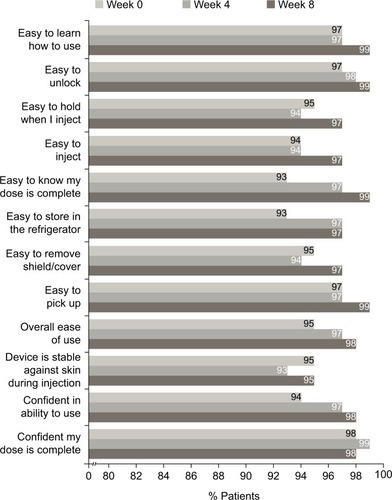
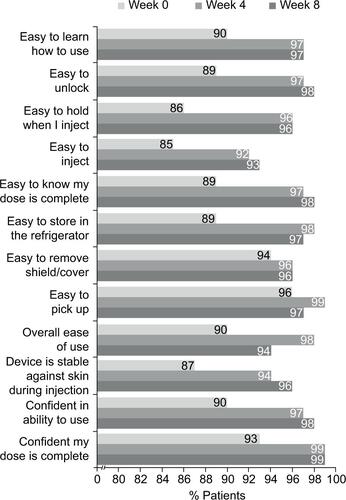
Figure S1 Patient responses to the SQAAQ weeks 0–4 and 8 (observed case data) – prefilled syringe group.
Notes: The proportion of patients that agreed or strongly agreed to each item of the SQAAQ is shown. Missing data were not imputed.
Abbreviation: SQAAQ, subcutaneous administration assessment questionnaire.
Video S1 Ixekizumab autoinjector.

