Figures & data
Figure 1 (A) Scanning electron microscope images of DMN, 500 µm in height and with 300 µm width at base. (B) A DMN, prior to application to the skin. (C, D) Representative optical coherence tomography (OCT) images showing DMN insertion in skin (C) and DMN dissolution in skin at time 0, 15 mins and 60 mins (D). Reproduced from Rodgers AM, McCrudden MT, Vincente-Perez EM, et al. Design and characterisation of a dissolving microneedle patch for intradermal vaccination with heat-inactivated bacteria: a proof of concept study. Int J Pharm. 2018;549(1–2):87–95. Creative commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.Citation10
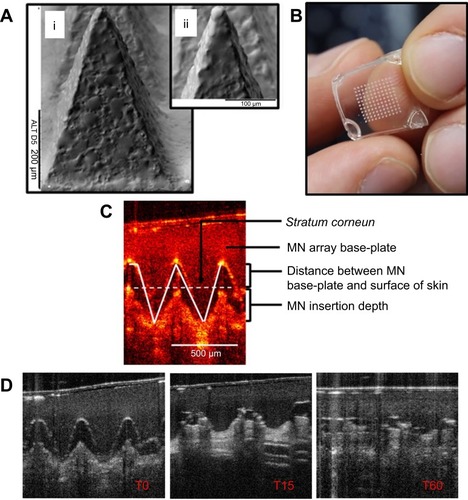
Figure 2 A schematic representation of the skin structure illustrating the different routes of administration, namely intramuscular, subcutaneous and ID injections. DMN penetrate the skin’s stratum corneum barrier reaching the viable epidermis, whereas the hypodermic needle punctures the skin into the subcutaneous and muscle tissue. Reproduced from Leone M, Mönkäre J, Bouwstra JA, Kersten GF. Dissolving Microneedle Patches for Dermal Vaccination. Pharm Res. 2017;34(11):2223-2240. Creative commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.Citation106
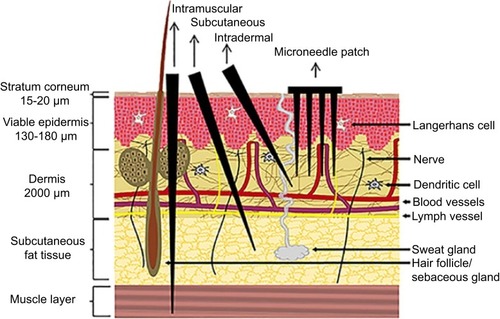
Table 1 Preclinical vaccine studies with DMN
Figure 3 Representative images of the DMN containing influenza vaccine employed during the Phase I trial. (A) Each DMN contained 100 microneedles, 650 µm in height, mounted on an adhesive backing and (B) the DMN was manually administered to the wrist, enabling self-administration by participants in the study. (C–D) Post insertion in the skin, the DMN dissolved thus delivering the influenza vaccine in the skin layers, represented here by a blue dye. (E) Some local reactions were evident in the skin post DMN insertion. (F) Local reactions associated with vaccination in the different groups are shown. Adapted from The Lancet, vol 190 (10095), Nadine G Rouphael, Michele Paine, Regina Mosley et al, The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial, pages 649-658, Copyright (2017), with permission from Elsevier.Citation18
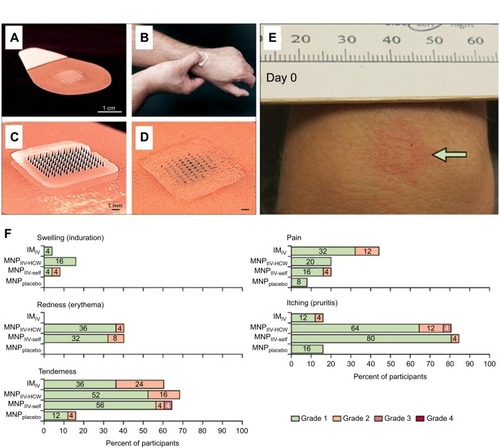
Figure 4 Representative images of DMN without dye (A) and with sulforhodamine B (B), observed using confocal microscopy. The theoretical DNA content per needle was calculated according to the volume of a conical shape, where R is the diameter and H is the height of the DMN (C). The DMN efficiently inserted in murine skin (D), as confirmed by histological analysis (E) (scale bar 100 µm). Bacterial counts in the lungs (F) and spleens (G) of immunized mice 4 weeks post challenge. Groups: Microneedle arrays without DNA (MNPWD), vector pVAX1 in saline as negative control, IMlow and IMhigh intramuscular injection groups given Ag85B DNA at a dose of 4.2 µg and 12.6 µg in 100-µL saline, MNPlow and MNP high given vaccine doses of 4.2 µg and 12.6 µg by administering one and DMN to each mouse. *p< 0.05, ***p< 0.001. Adapted from Vaccine, vol 36 (30), Qinying Yan, Zhigang Cheng, Houming Liu et al, Enhancement of Ag85B DNA vaccine immunogenicity against tuberculosis by dissolving microneedles in mice, pages 4471-4476, Copyright (2018), with permission from Elsevier.Citation73
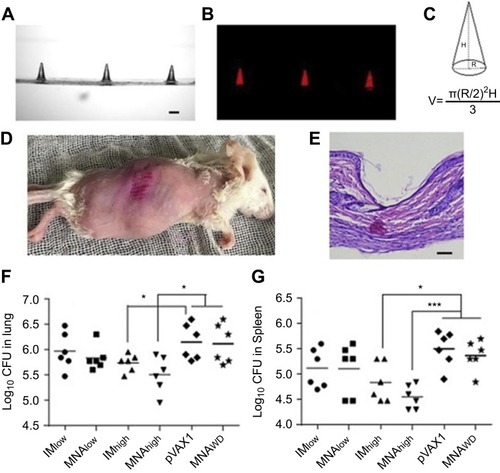
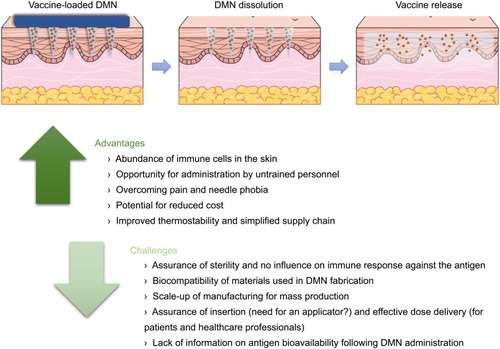
Figure 5 Schematic representation of the process of vaccine delivery using dissolving microneedle (DMN) arrays and summary of the main advantages and challenges associated with this potential vaccination strategy.