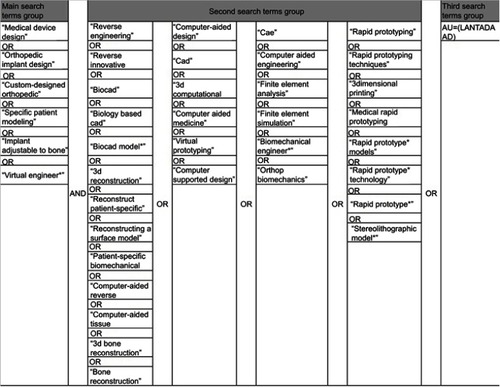
Figures & data
Figure 1 Research questions for exploratory literature review.
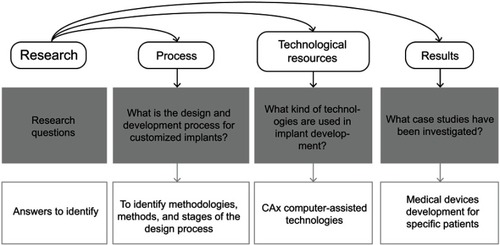
Figure 3 Frequencies of technology integration models observed.
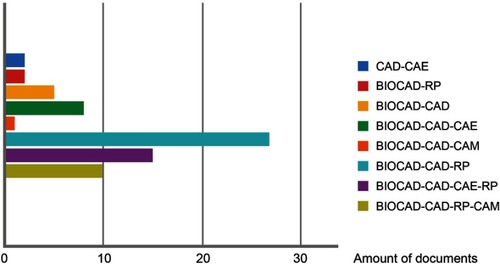
Figure 4 List of medical devices and technology integration models.
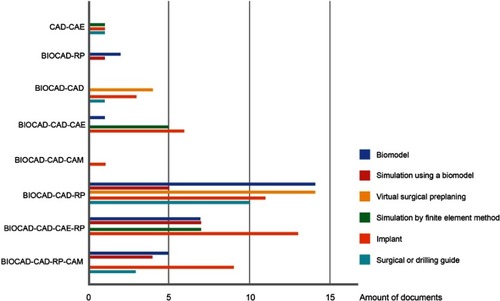
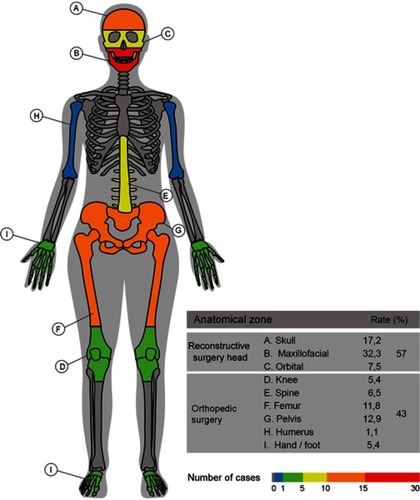
Table 1 Correlation among specific medical devices. Stronger correlations were among 0.75–0.99; moderate correlations for 0.4–0.74; low correlations for values close to 0; and inverse correlations for negative values
Figure 6 Relationships between the most frequent words in Tree of Science files.
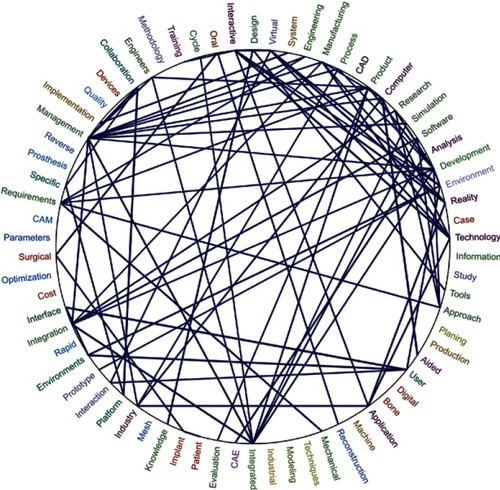
Figure 7 Coding of trends by networking.
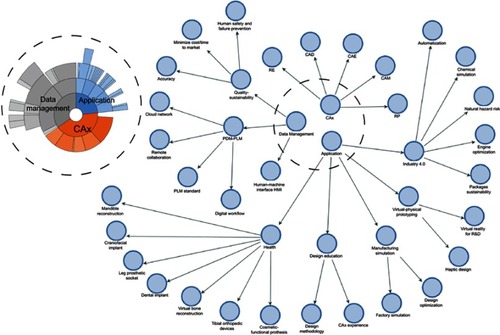
Figure 8 Requirements for design and development of specific medical devices.
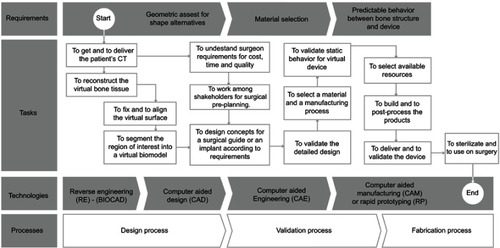
Table S1 Case studies categorized by Patient-specific Technology (PST), Patient-specific device (PSD), and anatomical zone